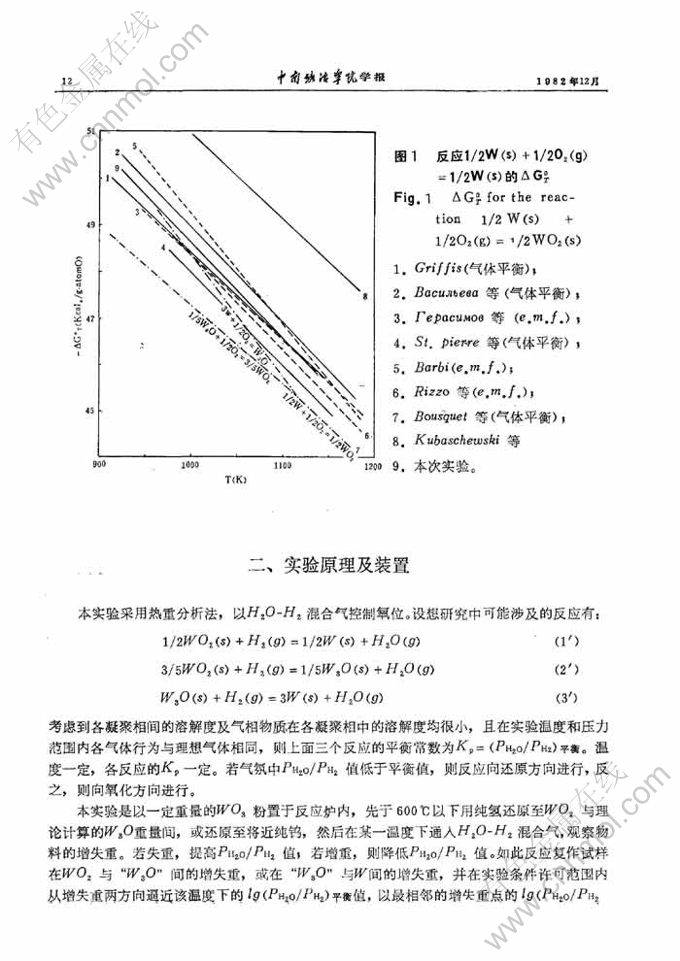
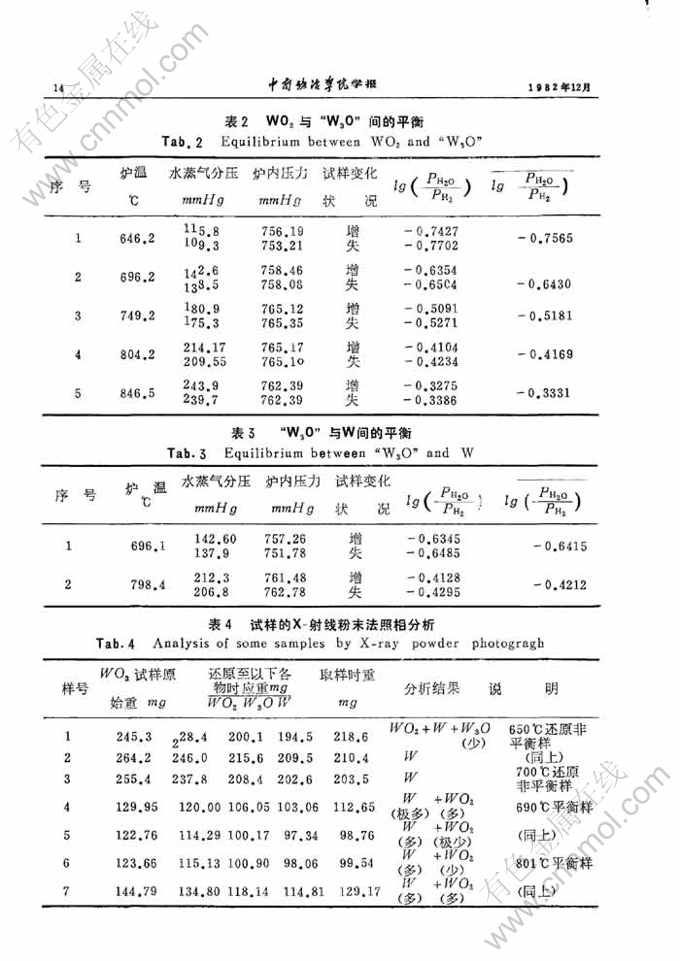
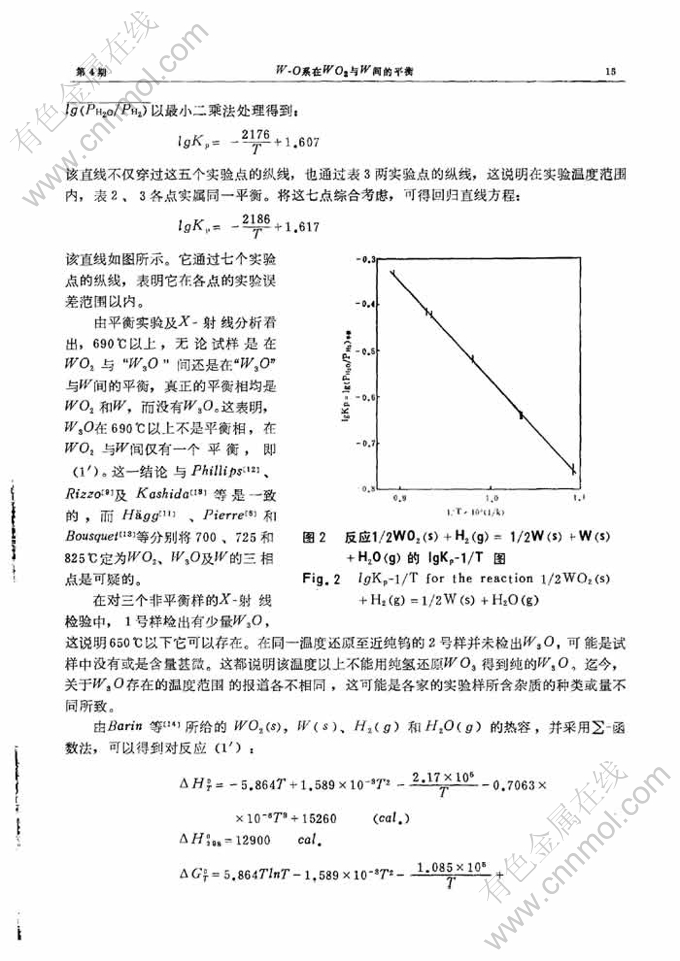
W-O系在WO2与W间的平衡
来源期刊:中南大学学报(自然科学版)1982年第4期
论文作者:黄慧民 陈新民
文章页码:11 - 17
关键词:标准生成热; 平衡值; 热重分析法; 平衡相; 气体平衡; 反应管; 实验点; 电动势法; 稳定平衡; 氢还原
摘 要:本文以热重分析法研究了650-850℃范围内W-O系在WO2与W间的平衡。结果表明,在690℃以上W3O不是一个平衡相。对于反应1/2WO2(s)+H2(g)=1/2W(s)+H2O(g),本文得出: lgKp=-(2186/T)+1.617 本文还得到WO2(s)的标准生成自由焓、标准生成热及标准熵。
Abstract:
In this paper a study was made on the equilibria of the W-O system be-tween WO2 and W in the temperature range 650--850℃. It was shown by thestudy that W3O is not an equilibrium phase above temperature 690℃. For thereaction
1/WO2(s) + H2(g) = 1/2W (s) + H2O(g)
the equilibrium constant-temperature relation was found to be
lgKp= -(2186/T)+ 1.617
and the standard free energy, the standard heat of formation and the stan-dard entropy of WO2 were presented as
△GT0= - 145000 - 7.837TlnT + 99.24T + 3.178×10-3T2 + + 2.17×105T-1- 0.7064×10-6T3±800 cal/mol.
△Hf2980= - 141.4 Kcal/mol.
S2980= 11.4 e.u.