
Electrical properties of La0.6Sr0.4Co1-yFeyO3 cathode material
JIANG Jin-guo(江金国),CUI Chong(崔 崇),LIU Jin-qiang(刘金强)
Department of Materials Science and Engineering,Nanjing University of Science and Technology,
Nanjing 210094, China
Received 20 April 2006; accepted 30 June 2006
Abstract: The electrical properties of La0.6Sr0.4Co1-yFeyO3 (LSCF, y=0-1.0) cathode materials were measured by DC four probes, X-ray photo-electron spectrum (XPS) was also introduced to determine the chemical state of Co, Fe ions in LSCF. It is found that the electrical conductivity of each sample has a maximum value with increasing temperature. XPS analysis shows that Co ion has three different chemical states, corresponding to two with Fe ion. The analyses indicates that the small-polaron hopping mechanism dominates the electron conduction at low temperature, while at high temperature, the three factors such as the thermally activated disproportionation of Co3+ ions into Co2+ and Co4+ pairs, the ionic compensation of oxygen vacancies formed at high temperatures, and Fe4+ ions charge compensation preferential to Co4+, all contribute to electrical conduction.
Key words: LaSrCoFeO3; oxygen vacancy; electrical conductivity; small-polaron; charge compensation; charge disproportionation
1 Introduction
Solid oxide fuel cell (SOFC) is an advanced energy-conversion device which can convert chemical energy in fuel into electric energy directly, and has many advantages, such as high energy-conversion efficiency environment friendly, non-pollution, which make it more and more attractive to worldwide researchers[1,2]. SOFC in previous research has to work at high temperature, which makes it difficult to put into practical use. Therefore, the recent trend of SOFC development is to decrease its work temperature and look for new materials operating at intermediate temperature[3]. La1-xSrxCo0.2- Fe0.8O3 (LSCF) perovskitetype composite oxide is an excellent ionic-electronic combined conductor, whose oxygen ion conduction can be attributed to doped various valent ions; in addition, it can exhibit electron conduction due to the change of transitional metal ions valent. The two factors above make it be a potential candidate for SOFC operating at intermediate temperature[4-8].
2 Experimental
La0.6Sr0.4Co1-yFeyO3 samples were prepared by solid phase reaction technique. The related information to the preparation and structure characterization of La0.6Sr0.4Co1-y FeyO3 samples can be found in Ref.[9]. The electrical conductivities between room temperature and 900 ℃ were measured by DC four probes, XPS test technique was also used to determine the chemical state of Co and Fe ions. In addition, the conduction mechanism is also taken into account.
3 Results and discussion
The XRD patterns of sintered La0.6Sr0.4Co1-yFeyO3 (y= 0.2, 0.4, 0.6, 0.8) samples are shown in Fig.1.
It can be found in Fig.1 that all formed phases are single perovskite-type structure, no other phases are detected. It is known that the radii of Co3+ ion, Fe3+ ion are 0.061 nm and 0.064 5 nm,respectively. As the content of Fe ion increases,the dimensions of the unit cell expand slightly, which results in the aberration of perovskite-type structure, but the effect is not so obvious as Sr-dope. Furthermore, the diffraction peak shifts slightly with increasing Fe ion content, but it doesn’t change the perovskite-type crystal structure fundamentally.
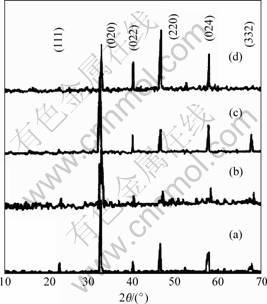
Fig.1 XRD patterns of La0.6Sr0.4Co1-yFeyO3 samples: (a) y=0.2; (b) y=0.4; (c) y=0.6; (d) y=0.8
The electrical conductivity(σ) in air for LSCF is plotted as a function of temperature in Fig.2. It shows that the electrical conductivity increases to a maximum then decreases as the temperature increases for each sample. The observed increment of the maximum of conductivity is weakened as the substitution of Fe for Co increases, i.e. the electrical conductivity increases obviously under small amount of substitution of Fe for Co, the trend to the electrical conductivity reduction is weakened as Fe substitution further increases. The phenomenon exhibits obviously during the period of low temperature in all curves.
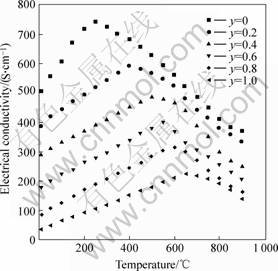
Fig.2 Electrical conductivity of La0.6Sr0.4Co1-yFeyO3 as function of temperature in air
Moreover, the temperature corresponding to maximum changes with various Co/Fe ratio from 200 to 700℃. The reason is that small-polaron hopping mechanism dominates at low temperature, the electrical conductivity is found to follow the relationship for the small-polaron hopping mechanism:
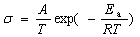 (1)
(1)
where A is a material constant containing the carrier concentration term; Ea is the activation energy for hopping conduction; T is the absolute temperature. This equation shows that the pre-exponential term will decrease with temperature increasery, but the exponential term will increase with temperature increasing. Accordingly, a maximum in electrical conductivity is expected to occur at Tmax=Ea/R, i.e. when the pre-exponential term starts to dominate the outcome of equation. The charge compensation of oxygen vacancy dominates electrical conduction at high temperature, and oxygen vacancy acts trap to catch carriers, resulting in the decrease of carriers concentration and mobility. As a result, the electrical conductivity decreases.
The measured electrical conductivities for various La0.6Sr0.4Co1-yFeyO3 compositions are plotted as ln(σT) versus T-1 in Fig.3. The slope for the linear portion of each data curve yields the activation energy for small-polaron conduction.
It is also noted (Fig.3) that most curves start to bend downward at some particular high temperatures, indicating that the small-polaron conduction is not the prevailing mechanism above these temperatures.
The calculated activation energy Ea values are listed in Table 1, the activation energies for all compositions are less than 0.1 eV.
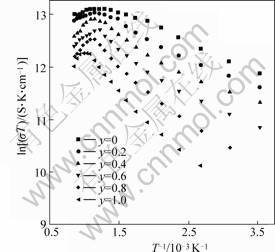
Fig.3 ln(σT) vs T-1 in air for LSCF with various Co/Fe ratios
Table 1 Activation energy for electrical conductivity vs compositions

According to the research reported by TAI et al[10], other electrical conduction mechanism may exist at high
temperature in La0.6Sr0.4Co1-yFeyO3 compositions except for the small-polaron hopping mechanism. The XPS results are in good accordance with the conclusions drawn by TAI et al subsequently.
The XPS results are shown in Figs.4 and 5, respectively. From the curves, it can be found that Co ion has three chemical states, corresponding to Co2+, Co3+ and Co4+, while Fe ion has only two chemical states as Fe3+ and Fe4+, all of which can confirm that the charge disproportionation of Co3+ ion occurs additionally.
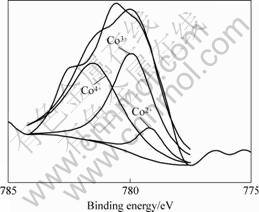
Fig.4 Co2p narrow zone spectra for La0.6Sr0.4Co0.2 Fe0.8O3 sample
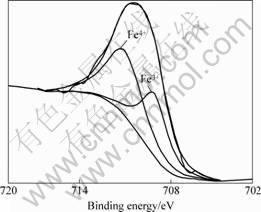
Fig. 5 Co 2p narrow zone spectra for La0.6Sr0.4Co0.2Fe0.8O3 Sample
3.1 Charge disproportionation of Co3+ ion
It has been shown that two Co3+ can be charged disproportionately into Co2+ and Co4+ by the thermally excited electron-transfer reaction as
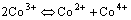 (2)
(2)
From the equation above we can know that the same amount of n- and p-type carriers can be formed due to the Co3+ disproportionation reaction, both of the two different type carriers can take part in electron conduction, which enhances the ability to electrical conduction. It has been reported that the Co3+ disproportionation reaction in LaCoO3 starts at 200 ℃, and the initiative reaction temperature decreases with Sr content increasing[4].
3.2 Ionic compensation of oxygen vacancies
In order to maintain charge neutrality in the system,
The doped Sr ions concentration 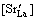 , B-site ions concentration
, B-site ions concentration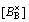 and oxygen vacancy concentration
and oxygen vacancy concentration
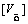 should follow the relationship as below
should follow the relationship as below
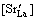 =
=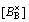 + 2
+ 2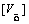 (3)
(3)
That is to say, the formation of an oxygen vacancy will prevent B-site ion hopping from trivalent to tetravalent, which will cause the reduction of the carriers concentration, thus the electrical conductivity decreases accordingly.
3.3 Charge compensation by Fe4+ ion in preference to Co4+ ion
During the period of low temperature, the charge compensation of oxygen vacancy can be ignored, the charge compensation in the system is conducted via the following two approaches:1) Co3+→Co4+; 2)Fe3+→Fe4+. Which approach on earth plays the role, we can find the answer in the curve of La0.6Sr0.4Co1-yFeyO3 electrical conductivity. Co is introduced in La0.6Sr0.4FeO3, the electrical conductivity doesn’t change apparently, which explains that the substitution of Co doesn’t alter the concentration of carriers in the system. On the other hand, when Fe is introduced to La0.6Sr0.4CoO3, the electrical conductivity decreases obviously, which indicates that Fe4+ ion is the primary carrier, since Fe has a weak ability to electrical conduction, the electrical conductivity reduces remarkably, i.e. in La0.6 Sr0.4 Co1-y-FeyO3 compositions, the charge compensation occurs by Fe3+→Fe4+ in preference to Co3+→Co4+.
4 Conclusions
1) The electrical conductivity for each La0.6Sr0.4Co1-y-
FeyO3 sample has a maximum as temperature increases; furthermore, the temperature corresponding to the maximum ascends as increasing Fe/Co ratio.
2) In La0.6Sr0.4Co1-yFeyO3 cathode materials, the electrical conduction appears to occur via a small-polaron hopping mechanism at low temperature. While at high temperature, except for small-polaron hopping mechanism, other factors such as: the thermally activated disproportionation of Co3+ ions into Co2+ and Co4+ pairs; the ionic compensation of oxygen vacancies formed at high temperatures; Fe4+ ions charge compensation preferential to Co4+, may contribute to the electrical conduction.
References
[1] TAKASHI T, RYOJI K, YOJI K, et al. New cathode materials for solid oxide fuel cell: ruthenium pyrochlore and perovskites[J].Journal of Electrochemical Society,1999,146(4):1273-1278.
[2] HUIJSMANS J P P , BERBEL F P F Van, CHRISTIE G M. New cathode materials for solid oxide fuel cell at low temperature[J].Journal of Power Science, 1998, 71 (3): 10-13.
[3] Teroka Y, Zhong H M, Obamoto K, et al. Mixed ionic-electronic conductivity of La1-xSrxCo1-yFeyO3 perovskite-type oxides [J].Material Research Bulletin, 1998, 23 (1): 51-55.
[4] TAI L W, NASRALLAH M M, ANDERSON H U, et al. Structure and electrical properties of La1-xSrxCo1-yFeyO3. Part 2. The system La1-xSrxCo0.2Fe0.8O3 [J]. Solid State Ionics, 1995,76(4): 273-283.
[5] CHEN C C, NARALLAH M M, ANDERSON H U. Immittance response of La0.6Sr0.4Co0.2Fe0.8O3 based electrochemical cells[J]. Journal of Electrochemical Society, 1995, 142(2): 491-498.
[6] RALPH J M, SCHOELER A C, RUMPELT M K.Materials for lower temperature solid oxide fuel cells [J]. Journal of Materials Science, 2001, 36(4): 1161-1168.
[7] KINDERMANN L, DAS D, NICKEL H, et al. Chemical compatibility of the LaFeO3 based perovskites (La0.6Sr0.4)zFe0.8M0.2O3 (z=0.9, 0.95; M=Cr, Mn, Co, Ni) with yttria stablized zirconia [J]. Solid State Ionics, 1996, 89: 215-224.
[8] DOSHI R, VON RICHARDS L, CARTER J D, et al. Development of solid oxide fuel cells that operate at 500 ℃[J]. Journal of Electrochemical Society, 2000, 147(5): 173-179.
[9] JIANG Jin-guo, CUI Chong, MA Rong. Preparation and characterization of La0.6Sr0.4Co1-yFeyO3 cathode materials[J]. Journal of Electronics Instrument and Measurements, 2003(suppl.): 607-609.(in Chinese)
[10] TAI L W, NASRALLAH M M, ANDERSON H U, et al. Structure and electrical properties of La1-xSrxCo1-yFeyO3. Part 1. The system La0.8Sr0.2 Co1-yFey O3[J]. Solid State Ionics, 1995,76(4): 259-271.
(Edited by LONG Huai-zhong)
Foundation item: Project (AB96055 ) supported by Nanjing University of Science and Technology
Corresponding author: JIANG Jin-guo; Tel: +86-25-84314956; E-mail: jiangjinguo@tom.com