文章编号:1004-0609(2014)06-1671-07
镁铁水滑石的表面化学性质及电化学行为
李 巧,曾虹燕,张治青,廖梦尘,黄清军,徐理华
(湘潭大学 化工学院,湘潭 411105)
摘 要:利用X射线衍射(XRD)、傅里叶变换红外光谱(FT-IR)、零电荷点pHPZC、循环伏安(CV)和电化学阻抗(EIS)等手段,研究镁铁水滑石(MgFe-LDHs)的表面化学性质,揭示其表面酸碱性与电化学性能的关系。结果表明:当Mg与Fe的摩尔比(n(Mg)/n(Fe))为3时MgFe-LDH (LDH-3)的结晶度最高,碱性最强,且远强于n(Mg)/n(Fe)为2的MgFe-LDH (LDH-2)和n(Mg)/n(Fe)为4的(LDH-4)的碱性。LDH-2的碱性略强于LDH-4的,但差异微小。层间CO32–与LDH层板金属离子主要形成单齿和双齿配位,其中,单齿配位体与强碱位点配位,形成Lewis碱位。表面碱性取决于Lewis强碱性位(CO32–单齿配位)的含量。用镁铁水滑石煅烧产物 (MgFe-LDOs) 修饰玻碳电极来研究其电化学性能。碱性越强(如LDO-3),其氧化还原可逆性及导电性能越好。
关键词:镁铁水滑石;表面酸碱性;配位;电化学性能;结晶度
中图分类号:O641.4 文献标志码:A
Surface chemical performances and electrochemical behavior of Mg-Fe hydrotalcite
LI Qiao, ZENG Hong-yan, ZHANG Zhi-qing, LIAO Meng-chen, HUANG Qing-jun, XU Li-hua
(School of Chemical Engineering, Xiangtan University, Xiangtan 411105, China)
Abstract: The surface chemical characteristics of the Mg-Fe hydrotalcites (MgFe-LDHs) were determined using X-ray diffractometry (XRD), Fourier transform-infrared spectrometer (FT-IR), point of zero charge (pHPZC), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The relationship between the surfaceacid-base and electrochemical properties of the MgFe-LDHs was clarified. The results show that the MgFe-LDH with molar ratio of Mg to Fe (n(Mg)/n(Fe)) being 3 (LDH-3) has the highest crystallity structure with symmetrical crystals, indicating that the surface basicity of the LDH-3 is the strongest. And its basicity is much higher than those of ones with n(Mg)/n(Fe) of 2 (LDH-2) and 4 (LDH-4). The basicity of LDH-2 is slightly stronger than that of LDH-4. The monodentate and bidentate coordinations are formed by the interlayer CO32– anions coordinating with the metal ions in the brucite-like layers. The monodentate ligand coordinates with the strong basic sites, then Lewis basic sites form. The basicity depends on the content of Lewis basic sites. Mg-Fe hydrotalcite calcined samples (MgFe-LDOs) were used to modify glassy carbon electrode to study its electrochemical properties. The stronger the basicity (such as those of the LDO-3) is, the better the oxidation-reduction reversibility and conductivity are.
Key words: Mg-Fe hydrotalcite; urfaceacid-base property; coordination; electrochemical property; crystallinity
水滑石(LDH)是一类具有层状结构的新型无机材料,属六方晶系,在载体、碱催化、吸附剂、离子交换和药物传输等领域有广泛的应用[1-4],高温焙烧得到的水滑石复合氧化物(LDO)具有比表面积大和强碱性的特点,其表面化学性质特别是微界面上酸碱性质也越来越受到人们的重视。国内外研究者通过改变制备方法、金属元素摩尔比、引入活性成分等来调整LDH表面化学性质[5-7]。用多种方法可表征水滑石表面化学性质,但各方法均有其局限性,需借助几种方法相互弥补完善[8]。虽然对LDH的表面酸碱性研究较多,但主要集中在表面酸碱性对其催化、吸附和离子交换性能的影响上[9-10],其表面酸碱性对电化学行为的影响机制研究相对缺乏。为此,本文作者采用XRD、FT-IR和零电荷点(pHPZC)等表征手段,研究不同Mg、Fe摩尔比的MgFe-LDH的表面化学性质,探明CO32-与LDH层板金属离子间的相互作用及其在表面的配位形式,并从Lewis酸碱理论讨论CO32-配位的本质,进而阐明其表面酸碱性与其电化学行为的关系。
1 实验
1.1 仪器与试剂
采用日本理学D/MAX-3C型X射线粉末衍射仪对样品物相进行表征,辐射源为Cu Kα,电压为40 kV,电流为30 mA,扫描速率为2(°)/min;采用PE公司生产的Specrum Qne B型傅里叶变换红外光谱仪进行FT-IR分析(KBr压片法),测定波数范围为4000~400 cm-1;采用梅特勒-托利多公司生产的5-2C型pH仪进行pH测定。所用试剂均为分析纯。采用CHI660C型电化学工作站(上海晨华仪器公司生产)进行电化学性能测试,三电极系统以自制修饰玻碳电极(GCE)为工作电极,饱和甘汞电极(SCE)为参比电极,铂电极为对电极。其中,将K4[Fe(CN)6]溶入pH值为9.0的磷酸缓冲液(PBS),制得0.5 mol/L K4[Fe(CN)6]溶液,作为电解质溶液,以饱和KBr液做盐桥,在室温下进行电化学性能测试。循环伏安(CV)扫描在-0.2~1.2 V电位范围和扫描速率100 mV/s下进行,每次实验均扫描20次。电化学阻抗谱测定频率范围为1~1×105 Hz,微扰电压为5 mV。
1.2 镁铁水滑石的制备
准确称取Mg(NO3)2·6H2O和Fe(NO3)3·9H2O(金属离子总量为0.5 mol),用去离子水配成Mg、Fe摩尔比分别为2、3和4的混合盐液A。另准确称取尿素,按尿素、NO3–摩尔比为4溶入A液中,滴加1 mol/L NaOH液调节A液的pH值为9.3。105 ℃油浴强力搅拌反应10 h,80 ℃晶化10 h,抽滤,洗涤,干燥,得Mg、Fe摩尔比为2、3和4的LDH,分别记为LDH-2、LDH-3和LDH-4。相应部分样品于500 ℃下煅烧5 h所得的LDO,分别记为LDO-2、LDO-3和LDO-4。
1.3 修饰电极的制备
依次分别用0.3和0.05 μm的Al2O3粉将GCE(直径为3 mm)抛光成镜面,用无水乙醇超声洗涤3 min,去离子水淋洗干净,晾干预处理GCE。在10 mL去离子水中加入20.0 mg LDO,超声分散1 h,移取10 μL滴涂至预处理GCE表面,红外灯下晾干约3 min,得Mg、Fe摩尔比分别为2、3和4的LDO修饰工作电极,分别记为LDO-2/GCE、LDO-3/GCE和 LDO-4/GCE。
1.4 零电荷点的测定
取LDH-2、LDH-3和LDH-4样品,依次分别加入浓度为0.100、0.010和0.001 mol/L的KNO3液中至样品浓度为1.0 g/L,混合液在25 ℃平衡24 h。分别用0.001 mol/L的KOH溶液滴定,记录滴加KOH溶液体积以及平衡时pH值(pH值在5 min内无明显变化视为平衡)。其中,KOH溶液体积应扣除滴定相应空白对照所消耗的KOH溶液体积。根据相应的KOH溶液体积和pH值,计算H+和OH–的吸附量。以ГOH–和ГH+差值 (ГOH– -ГH+)对pH值绘制滴定曲线,不同浓度曲线交点对应的pH值即为该样品的零电荷点pHZPC。
1.5 电极活化面积的测定
采用电位阶跃计时库仑法(CC法)计算修饰电极活化面积,实验条件与循环伏安同。以0.1 mmol/L的K3[Fe(CN)6]液为模型化合物(1.0 mol/L KCl电解质液中扩散系数 D为7.6×10-6 cm2/s)[11-12],根据Anson方程[13]:
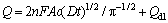 (1)
(1)
式中:Q为时间t时的电量;n为电子转移数;F为法拉第常数;A为电极面积(cm2);c为K3[Fe(CN)6]溶液的浓度(mol/L);Qdl为双电层的充电电量。
2 结果与讨论
2.1 镁铁水滑石的表征
2.1.1 LDH的XRD谱分析
图1所示为不同Mg、Fe摩尔比LDH的XRD谱。由图1可知,所有样品均具典型水滑石层状结构。LDH-3的各衍射峰强度最高、对称性良好,峰型最为尖锐、几乎无杂峰(基线最平稳),结晶度最高;而LDH-2各衍射峰宽化,基线漂移,晶体结构规整度降低,结晶度略低;LDH-4有Mg2(OH)2CO3杂峰出现,可能是随Mg、Fe摩尔比增大,物相中Mg2+含量过高,从而形成Mg2(OH)2CO3晶相,因此,结晶度最低。分析结果表明LDH-3的结晶度最高,晶相单一,结构规整。

图1 不同Mg、Fe摩尔比LDHs的XRD谱
Fig. 1 XRD patterns of LDHs with different molar ratios of Mg to Fe
2.1.2 LDH的FT-IR光谱分析
图2所示为不同Mg、Fe摩尔比LDHs的FT-IR光谱。由图2可知,所有样品均具典型水滑石特征红外吸收峰。3500~3600 cm-1处的吸收峰归属于水滑石层板—OH的伸缩振动,1200~1800 cm-1区间则归属于层间CO32–的振动吸收峰,400~1200 cm-1区间归属于CO32-和层间晶格Mg—O和Fe—O的振动吸收峰。
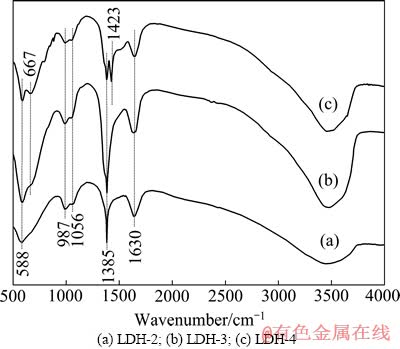
图2 不同Mg、Fe摩尔比LDHs的FT-IR光谱
Fig. 2 FT-IR spectra of LDHs with different molar ratios of Mg to Fe
1630和1423 cm-1附近归属于CO32–反对称伸缩振动吸收峰[14]。1385 cm–1附近归属于CO32–的反对称伸缩振动,与自由CO32–的反对称伸缩振动(1423 cm–1)相比,向低波数方向发生较大偏移,说明层间CO32–离子与层板金属离子配位或与H2O及层板—OH形成氢键。1056 cm–1附近吸收峰对应LDH层间CO32–的对称伸缩振动,987和667 cm–1附近处吸收峰分别为CO32–面外弯曲振动和面内弯曲振动,也可能归属于层间晶格Mg—O和Fe—O的振动吸收峰(Mg—O,Fe—O)。588 cm–1归属于Mg—O和Fe—O的晶格振动。
自由态CO32–属于D3h点群,有4类振动模式,在987、667和1423 cm–1附近振动模式具红外活性,其中在 1423 cm–1处为二重简并峰,在1056 cm–1处虽为非红外活性,但晶体场中1056 cm–1处吸收峰可显现(见图2)。配位后的CO32–,其对称性降低,简并振动变为非简并振动,相应吸收裂解。配位CO32–主要以单齿(配位强碱位)、双齿(配位中等碱位)以及桥联(配位弱碱位)配位形式存在[15]。所有样品在1423 cm–1处的自由态CO32–反对称伸缩振动单峰一分为二,形成明显吸收峰,可归属为与层板金属离子配位的表面CO32– (单齿和双齿)的振动峰。1200~1800 cm–1为CO32–不对称振动吸收区间,可利用出现在该区间谱带振动峰的分裂程度来辨别双齿配位、单齿配位或桥联配 位。在1360~1400 cm–1和1510~1560 cm–1处的吸收峰归属于单齿CO32–振动峰,而1320~1340和1610~1630 cm–1处的吸收峰则归属于双齿CO32–振动峰,1650 cm–1附近的归属于桥联配位的HCO3–中C—O—C的反对称伸缩振动峰[16]。
为了更深入研究LDHs的表面化学性质,采用PeakFit软件对不同Mg、Fe摩尔比LDH样品的FT-IR在1200~1800 cm–1区间光谱带进行去卷积及高斯函数拟合(R2>0.997),对CO32–配位的变化进行研究,结果见图3和表1。由图3和表1可知,3种样品在1423 cm–1 附近均存在自由CO32–反对称伸缩振动吸收峰,说明样品层间还存在自由CO32–,其中,LDH-4的自由CO32–含量最高,LDH-2的次之,而LDH-3的明显低于前两者。CO32–单齿、双齿配体分别是与层板上强和中等碱位点配位,而CO32–桥联配体是与层板上弱碱位点配位[15]。因而可用其单和双齿配体的相对含量衡量样品碱性强弱。LDH-3的总碱位(单齿和双齿)含量最高(70.66%),LDH-2的其次(40.85%),LDH-4的最低(35.99%),特别是LDH-3的强碱位(单齿CO32–)含量最高(62.61%),LDH-2的其次(25.11%),而LDH-4的最低(20.30%),其中,LDH-2和LDH-4的总碱位和强碱位含量差异较小(LDH-2的略高于LDH-4的)。上述分析表明,不同Mg、Fe摩尔比LDHs的表面化学性质即表面碱性不同,因为水滑石的碱性主要由强碱位(单齿CO32–)决定,所以LDH-3的碱性最强,且LDH-2的碱性略强于LDH-4的碱性。

图3 LDHs的FT-IR光谱在1200~1800 cm–1范围的拟合图
Fig. 3 Fitted curves of FT-IR spectra for LDHs in region of 1200-1800 cm–1
表1 LDHs在1200~1800 cm–1处子峰位置、相对面积及归属
Table 1 Infrared band positions, areas and assignments in 1200-1800 cm-1 region of LDHs

2.1.3 LDH表面酸碱性(零电荷点pHPZC)
pHPZC是表征水滑石表面酸碱性的一个参数,是指水溶液中固体表面净电荷为零时的pH值。图4所示为LDH样品的PT曲线。由图4可知,LDH-2、LDH-3和LDH-4的零电荷点pHZPC分别为11.2、11.8和11.1,且(ГOH– -ГH+)ZPC为正值,表明样品表面均呈碱性,带永久正电荷[17],其碱性由大到小的顺序为LDH-3、LDH-2、LDH-4(LDH-2的碱性略高于LDH-4的)。可见,LDH-3的零电荷点有所增加,说明LDH-3的表面碱性最强,这一顺序也与LDH样品的FT-IR分析结果一致,这也进一步证明了LDH-3具有最强和更多的活性碱性位。另外,LDO-2、LDO-3和LDO-4的pHZPC值经测定分别为11.3、12.0和11.0,与相应的LDH的pHPZC值相差微小,可能是LDO零电荷点测定在水中进行,LDO由于“结构记忆效应”完全恢复原有层板晶体结构,即恢复至LDH,使之恢复原有LDH表面化学性质的缘故。为此,推论通过调节Mg、Fe摩尔比可实现对水滑石及其煅烧产物酸碱性的调节,这对需不同表面酸碱性水滑石材料无疑是极为重要的。
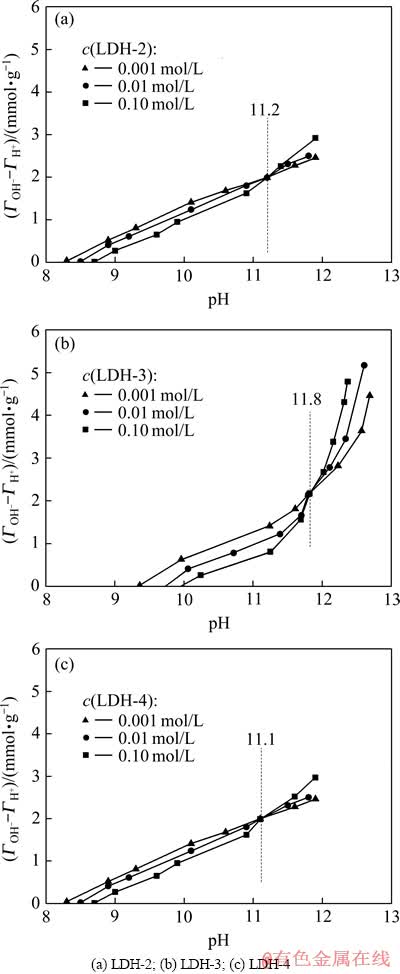
图4 KNO3溶液中含不同Mg、Fe摩尔比的LDHs的电势滴定曲线
Fig. 4 Potentiometric titration curves of LDHs with different molar ratios of Mg to Fe in KNO3 solution
2.2 LDO的电化学性能
LDO作为电化学活性材料具较弱的导电能力,且其电化学性质与其表面化学性能相关,为了更深入地研究LDO表面化学性质,考察不同Mg、Fe摩尔比的LDO修饰玻碳电极的电化学行为,探讨导电性能与其表面化学性质的关系,本文作者对3个样品进行循环和交流阻碍测试,结果见图5和6。
2.2.1 LDO/GCE的伏安行为
3个样品在20次扫描过程中的电流响应值均比较稳定,曲线变化微小而相互重叠,难以辨清(数据未显示),说明其电化学稳定性均较好。再则,LDO-2/GCE和LDO-4/GCE间电流响应值差异微小,为更清楚地说明不同摩尔比样品间的差异,仅以LDOs/GCE在0.5 mol/L K4[Fe(CN)6]溶液中第20次扫描伏安数据作图(见图5)。所有电极的循环伏安曲线均有一对氧化还原峰,其阴极峰电位φpc、阳极峰电位φpa以及两峰电位差△φp,阴极峰电流Ipc、阳极峰电流Ipa值见表2。LDO-3/GCE的氧化还原峰电流明显大于LDO-2/GCE和LDO-4/GCE的,而LDO-2/GCE和LDO-4/GCE的几乎无差异。各电极Ipa/Ipc ≈ 1,即其阳极峰电流Ipa与阴极峰电流Ipc近似,说明氧化还原反应可逆性好。LDO-2/GCE的△φp略低于LDO-4/GCE的△φp,但前两者明显大于LDO-3/GCE的,说明LDO-3/GCE的可逆性更好,极化程度更大。电极活化面积A(R2>0.9997)(见表2)越大,电极活性位点越多,氧化还原反应就越灵敏。LDO-2/GCE和LDO-4/GCE的A几乎相等(LDO-2/GCE的略大于LDO-4/GCE的),LDO-3/GCE的活化面积A明显大于前两者的,即LDO-3/GCE可吸附更多的[Fe(CN)6]4–进行电子转移,导电性更强。LDO-3的表面碱性最强,其具最多的强给电子能力的含O2–官能团,从而导致在碱性电解液中LDO-3/GCE的活化面积明显增大,导电性增强。
2.2.2 LDO/GCE的交流阻抗分析
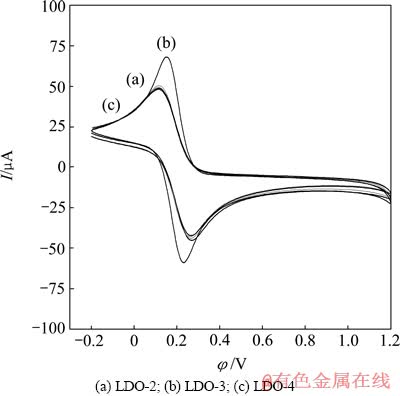
图5 LDO/GCE在0.5 mol/L K4[Fe(CN)6]溶液中溶解的循环伏安曲线
Fig. 5 Voltammetric cycle curves of LDOs/GCE in 0.5 mol/L K4[Fe(CN)6] solution
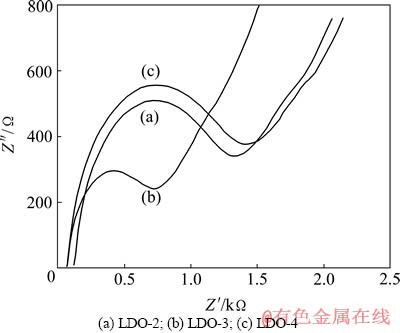
图6 在0.5 mol/L K4[Fe(CN)6]溶液中LDOs/GCE的交流阻抗图
Fig. 6 EIS of LDOs/GCE in 0.5 mol/L K4[Fe(CN)6] solution
表2 LDOs/GCE的循环伏安参数
Table 2 Cyclic voltammeter parameters of LDOs/GCE

电化学阻抗谱(EIS)是研究材料性质、化学(特别是电化学)过程、界面结构和表面化学性质的有力工具[18]。图6所示为LDO/GCE工作电极循环10次的EIS图。低频区的圆弧随极化程度的增大而逐渐变小,该区阻抗主要对应于氧化还原反应的电荷传递阻抗。由图6可知,低频区3样品电极的EIS均呈现一半圆,LDO-2/GCE的半圆弧半径略小于LDO-4/GCE的,但LDO-3/GCE的远小于前两者的半径,表明LDO-3/GCE的阻抗大大降低,反应速度加快[18-19]。这是因为LDH-3表面具有强给电子能力,形成负电中心,增大了导电性,使阻抗弧半径显著减小,阻抗值显著降低,氧化还原反应得到大幅加速。这一结果表明,LDO-3具有最低的电化学电阻和最为优良的电化学电容行为,LDO-2次之,而LDO-4仅微弱于LDO-2,这与图3所示的循环伏安曲线所得的结果一致。
综上所述,不同Mg、Fe摩尔比LDH的结晶度不同,基本上体现了结晶度越高,该类碱中心强度越强,表面含氧碱性官能团越多,电化学性能越好的特征,即高结晶度能够显著增加LDH的强碱位中心,这与文献[20]报道结果一致。
2.3 表面酸碱性与电化学行为的关系
水滑石是一类由中心原子和配位原子通过氧原子桥联方式进行空间组合的多氧簇金属配合物,它是一种酸碱性与氧化还原性兼备的双功能无机材料。金属氧化物与金属不同,其表面具有较高能量,其电化学性能取决于其表面化学性能,特别是其表面酸碱性。MgFe-LDH的结晶度与CO32–和表面金属离子的配位形式有关,结晶度越高,CO32–与层板金属离子形成强碱性位的单齿配位越多,其碱性越强,表面含O2–越多,也即给电子能力越强。根据Lewis酸碱理论,CO32–的单齿配位为Lewis碱性位。Lewis碱的中心为氧负离子,其负电荷的大小,即供电子能力,决定了碱性的强弱,即负电荷越多,碱性越强,也即表面O2–的原子核周围电子密度越高,碱性越强。LDO在电解液接触中,由于“结构记忆效应”完全恢复原有层板晶体结构,CO32–嵌入层间,LDO与与水分子接触后表面立即与水结合,相界面发生荷电离子转移,使得LDO表面羟基化,恢复形成LDH。表面—OH基团可离子化,CO32–与层板金属离子进行单齿和双齿配位而表现出Bronsted和Lewis酸碱性,其中,双齿配位为Bronsted的弱碱位而单齿配位是Lewis强碱[7, 21],并受界面静电场强度影响。在电场作用下,LDH表面电子发生振动获得能量,克服势垒发生跃迁,跃迁谱带向低能区移动。不同Mg、Fe摩尔比LDHs的表面化学性质即表面碱性不同。LDH-3的碱性最强,即O2–周围电子密度最大,大量的电子克服势垒发生跃迁,其修饰的电极导电性也就最强。而低结晶度的水滑石表面CO32–与层板金属离子配位所形成的O2-密度低,其电化学性能降低。
3 结论
1) 不同Mg、Fe摩尔比(2:1~4:1)MgFe-LDH中LDH-3的结晶度最高,碱性最强,LDH-2的结晶度次之,碱性减弱,而LDH-4结晶度最差,碱性最弱,其中LDH-2和LDH-4的碱性相差微弱。
2) FT-IR表征表明,层间CO32–主要以单齿和双齿配位形式存在,表面碱性取决于Lewis强碱性位碱位(单齿CO32–),LDH-3的强碱位最多,且总碱位(单齿和双齿)含量最高,而LDH-2的略多于LDH-4的,但差异微小。pHPZC值和与表面酸性含氧官能团的数量呈负相关,即pHPZC值越大,其表面碱性越强,这进一步证明了结晶度最高的LDH-3的碱性最强。
3) CV和EIS测试表明,LDO的电化学性能与其表面酸碱度有关,LDO-3的碱性最强。CV测试表明LDO-3的氧化还原可逆性及导电性能最好。
REFERENCES
[1] COQ B, TICHIT D, RIBET S. Co/Ni/Mg/Al layered double hydroxides as precursors of catalysts for the hydrogenation of nitriles: hydrogenation of acetonitrile[J]. Journal of Catalysis, 2000, 189(1): 117-128.
[2] ANER S M, WANDELER R,  U, TRUJILLANO R, LABAJOS F M, RIVES V. Heterogeneous coupling of phenylethyne over Cu-Mg-Al mixed oxides: Influence of catalyst composition and calcination temperature on structural and catalytic properties[J]. Journal of Catalysis, 1997, 169(1): 1-12.
U, TRUJILLANO R, LABAJOS F M, RIVES V. Heterogeneous coupling of phenylethyne over Cu-Mg-Al mixed oxides: Influence of catalyst composition and calcination temperature on structural and catalytic properties[J]. Journal of Catalysis, 1997, 169(1): 1-12.
[3] LAZARIDIS N K, ASOUHIDOU D D. Kinetics of adsorptive removal of chromium (Ⅵ) from aqueous solutions by calcined Mg-Al-CO3 hydrotalcite[J]. Water Research, 2003, 37(12): 2875-2882.
[4] KHAN A I, O'HARE D. Intercalation chemistry of layered double hydroxides: recent developments and applications[J]. Journal of Materials Chemistry, 2002, 12(11): 3191-3198.
[5] 吴 雁, 王 豪, 钟 婷, 张 灵, 胥利蛟. 不同方法合成的镁铝水滑石的表征及其催化原油酯化脱酸性能[J]. 石油学报: 石油加工, 2011, 27(5): 687-693.
WU Yan, WANG Hao, ZHONG Ting, ZHANG Ling, XU Li-jiao. Characterization of MgAl hydrotalcites prepared by various methods and their catalytic activities for esterification to remove acid from crude oil[J]. Acta Petrolei Sinica: Petroleum Processing Section, 2011, 27(5): 687-693.
[6] 李丽芳, 侯万国, 戴肖南, 刘春霞. Zn-Al类水滑石零净电荷点及等电点研究[J]. 化学学报, 2004, 62(4): 429-432.
LI Li-Fang, HOU Wan-guo, DAI Xiao-nan, LIU Chun-xia. Studies of the point of zero net charge and isoelectric point of Zn-Al hydrotalcite-like compounds[J]. Acta Chimica Sinica, 2004, 62(4): 429-432.
[7] 张业新, 苏庆运, 王仲鹏, 高希彦, 张昭良. 钾对镁铝水滑石复合氧化物的表面改性[J]. 物理化学学报, 2010, 26(4): 921-926.
ZHANG Ye-xin, SU Qing-yun, WANG Zhong-peng, GAO Xi-yan, ZHANG Zhao-liang. Surface modification of Mg-Al hydrotalcite mixed oxides with potassium[J]. Acta Physico-Chimica Sinica, 2010, 26(4): 921-926.
[8] 臧 杨, 郝晓刚, 王忠德, 张忠林 刘世斌. 碳纳米管/聚苯胺/铁氰化镍复合膜的电化学共聚制备与电容性能[J]. 物理化学学报, 2010, 26(2): 291-298.
ZANG Yang, HAO Xiao-gang, WANG Zhong-de, ZHANG Zhong-lin, LIU Shi-bin. Copolymerization and capacitive performance of composite carbon nanotubes/polyaniline/nickel hexacyanoferrate films[J]. Acta Physico-Chimica Sinica, 2010, 26(2): 291-298.
[9] SCAVETTA E, BERRETTONI M, GIORGETTI M, TONELLI D. Electrochemical characterisation of Ni/Al-X hydrotalcites and their electrocatalytic behaviour[J]. Electrochimica Acta, 2002, 47(15): 2451-2461.
[10] BALLARIN B, SEEBER R, TONELLI D, VACCARI A. Electrocatalytic properties of nickel (Ⅱ) hydrotalcite-type anionic clay: application to methanol and ethanol oxidation[J]. Journal of Electroanalytical Chemistry, 1999, 463(1): 123-127.
[11] 金荣荣, 李丽芳, 徐雪峰, 连英惠, 赵 凡. 水滑石负载的钯纳米粒子对水合肼的电催化氧化[J]. 物理化学学报, 2012, 28(8): 1929-1935.
JIN Rong-rong, LI Li-fang, XU Xue-feng, LIAN Ying-hui, ZHAO Fan. Layered double hydroxide supported palladium nanoparticles for electrocatalytic oxidation of hydrazine[J]. Acta Physico-Chimica Sinica, 2012, 28(8): 1929-1935.
[12] 李俊华, 邝代治, 冯泳兰, 刘梦琴, 唐斯萍, 邓培红. 基于银纳米粒子/石墨烯复合薄膜制备TNP电化学传感器[J]. 无机化学学报, 2013, 29(6): 1157-1164.
LI Jun-hua, KUANG Dai-zhi, FENG Yong-lan, LIU Meng-qin, TANG Si-ping, DENG Pei-hong. Preparation of TNP electrochemical sensor based on silver nanoparticles/graphene oxide nanocomposite[J]. Chinese Journal of Inorganic Chemistry, 2013, 29(6): 1157-1164.
[13] 赵 鹏, 方慧珏, 薛 腾, 亓西敏, 陆嘉星. 计时电量法求NiCl2(bpy)3在DMF中的扩散系数和速率常数[J]. 物理化学学报, 2005, 21(11): 1235-1239.
ZHAO Peng, FANG Hui-jue, XUE Teng, QI Xi-min, LU Jia-xing. Diffusion coefficients and rate constants of NiCl2(bpy)3 in DMF determined by chronocoulometry[J]. Acta Physico- Chimica Sinica, 2005, 21(11): 1235-1239.
[14] 祁敏佳, 宋兴福, 杨 晨, 孙淑英, 于建国. 微波对碱式碳酸镁结晶过程的影响[J]. 无机化学学报, 2012, 28(1): 1-7.
QI Min-jia, SONG Xing-fu, YANG Chen, SUN Shu-ying, YU Jian-guo. Effect of microwave on the crystallization process of hydromagnesite[J]. Chinese Journal of Inorganic Chemistry, 2012, 28(1): 1-7.
[15]  X, SALAGRE P, CESTEROS Y. Study of alkaline-doping agents on the performance of reconstructed Mg-Al hydrotalcites in aldol condensations[J]. Applied Catalysis A: General, 2005, 281(1/2): 191-198.
X, SALAGRE P, CESTEROS Y. Study of alkaline-doping agents on the performance of reconstructed Mg-Al hydrotalcites in aldol condensations[J]. Applied Catalysis A: General, 2005, 281(1/2): 191-198.
[16] DI COSIMO J I,  C R. Synthesis of α, β-unsaturated ketones over thermally activated Mg-Al hydrotalcites[J]. Applied Clay Science, 1998, 13(5): 433-449.
C R. Synthesis of α, β-unsaturated ketones over thermally activated Mg-Al hydrotalcites[J]. Applied Clay Science, 1998, 13(5): 433-449.
[17] 金志琳, 侯万国, 张春光, 孙祉伟, 杨亲正, 孙德军, 戴国亮. Mg-Al-NO3层状双金属氢氧化物电性质研究[J]. 化学学报, 2003, 61(8): 1208-1212.
JIN Zhi-lin, HOU Wan-guo, ZHANG Chun-guang, SUN Zhi-wei, YANG Qing-zheng, SUN De-jun, DAI Guo-liang. Studies on electric property of Mg-Al-Nitrate layered double hydroxide [J]. Acta Chimica Sinica, 2003, 61(8): 1208-1212.
[18] 刘 鸿, 吴 鸣, 吴合进, 孙福侠, 郑 云, 李文钊. 氢处理二氧化钛的光催化性能及电化学阻抗谱[J]. 物理化学学报, 2001, 17(3): 286-288.
LIU Hong, WU Ming, WU He-jin, SUN Fu-xia, ZHENG Yun, LI Wen-zhao. Photocatalytic activity and electrochemical impedance spectroscopy of TiO2 thermally treated by hydrogen[J]. Acta Physico-Chimica Sinica, 2001, 17(3): 286-288.
[19] 褚有群, 韩文锋, 童少平, 马淳安. 活性炭表面性质对氧气扩散电极电催化性能的影响[J]. 催化学报, 2006, 27(2): 183-188.CHU
You-qun, HAN Wen-feng, TONG Shao-ping, MA Chun-an. Effects of surface nature of activated carbon on electrocatalytic performance of oxygen diffusion electrodes[J]. Chinese Journal of Catalysis, 2006, 27(2): 183-188.
[20] 肖慧明, 王建明. Al含量对α-Ni(OH)2结构及其电化学性能的影响[J]. 无机材料学报, 2004, 19(3): 463-470.
XIAO Hui-ming, WANG Jian-ming. Influence of Al content on the structure and electrochemical performance of Al-substituted α-Ni(OH)2 [J]. Journal of Inorganic Materials, 2004, 19(3): 463-470.
[21]  C R, DI COSIMO J I. Effect of the chemical composition on the catalytic performance of MgyAlOx catalysts for alcohol elimination reactions[J]. Journal of Catalysis, 2003, 215(2): 220-233.
C R, DI COSIMO J I. Effect of the chemical composition on the catalytic performance of MgyAlOx catalysts for alcohol elimination reactions[J]. Journal of Catalysis, 2003, 215(2): 220-233.
(编辑 陈卫萍)
基金项目:湖南省自科基金重点项目(12JJ2008);湖南省高校创新平台开放基金项目(12K048);2012年湖南省大学生创新性实验计划资助项目(2012)
收稿日期:2013-10-21;修订日期:2014-03-28
通信作者:曾虹燕,教授,博士;电话:0731-58298175;E-mail: hyzeng@xtu.edu.cn