超细磷酸锌钙粉体的制备与性能
谢飞,曹有名,雷周桥,周心其,雷敏娟
(广东工业大学 材料与能源学院,广东 广州,510006)
摘要:采用正交实验设计,以Ca(OH)2,ZnO和H3PO4为原料,通过水热法合成出超细磷酸锌钙粉体。借助于动态光散射粒度分析仪、扫描电镜对产物的粒度和形貌进行分析表征,通过XRD图谱对反应机理进行研究,同时通过TG图谱对产物失水过程进行分析。实验结果表明:最佳反应条件是:Ca与Zn摩尔比n(Ca):n(Zn)=1:2,反应温度为80 ℃,反应时间为12 h,OP-10为表面活性剂;得到的产物颗粒大小均匀,粒度为1~2 μm;最终产物CaZn2(PO4)2·2H2O是通过中间产物Ca[Zn(OH)3]2·2H2O与磷酸反应而生成的。CaZn2(PO4)2·2H2O分子的失水过程分为2个阶段,第1阶段失水温度为95~393 ℃,第2阶段失水温度为403~553 ℃。
关键词:水热合成法;超细磷酸锌钙粉体;反应机理;失水过程
中图分类号:TQ628.3 文献标志码:A 文章编号:1672-7207(2012)04-1295-04
Preparation and properties of ultrafine CaZn2(PO4)2 powder
XIE Fei, CAO You-ming, LEI Zhou-qiao, ZHOU Xin-qi, LEI Min-juan
(Faculty of Materials and Energy, Guangdong University of Technology, Guangdong, 510006, China)
Abstract: Based on the orthogonal experiment, ultrafine CaZn2 (PO4)2 powder was prepared using calcium hydroxide, zinc oxide and phosphoric acid as main raw materials by hydrothermal reaction method. The particle size and morphologies of samples were characterized by DLS and SEM, the reaction mechanism was investigated by XRD, and the decomposition process of products was revealed with thermal analytical techniques. The results show that optimum reaction conditions are as follows. The molar ratio of reactant n(Ca):n(Zn) is 1:2, the reaction temperature is 80°C, the reaction time is 12 h, and the surfactant is OP-10. The diameter distribution of the product is homogeneous, and the average particle diameter is about 1~2 μm. The final product CaZn2(PO4)2·2H2O is synthesized by the intermediate production Ca[Zn(OH)3]2·2H2O and phosphoric acid. The CaZn2 (PO4)2·2H2O molecular is decomposed to CaZn2 (PO4)2 in two dehydration reactions stage: the first stage of dehydration temperature is from 95 ℃ to 393 ℃, and the second stage of dehydration temperature is from 403 ℃ to 553 ℃.
Key words: hydrothermal synthesis; ultrafine CaZn2 (PO4)2 powder; reaction mechanism; dehydration process
磷酸锌作为一种性能优良的无毒防锈颜料,能够与酚醛树脂、环氧树脂、中油度醇酸和氨基甲酸树脂等配制防腐蚀涂料,是目前用量最大、应用最广泛的生态型防锈颜料。由于传统合成的磷酸锌产品存在活性低、原料成本高等问题致使其无法全面取代传统的铬铅类有毒防锈颜料[1-3]。为了提高磷酸锌的活性和降低成本,拓宽其应用领域,对其改性或开发出具有高活性的磷酸锌就显得相当重要。用阳离子(如Ca2+,K+,Al3+等),或阴离子(如SiO44-,MoO43-和OH-等),或同时增加阴离子和阳离子部分或全部取代锌,制成改性磷酸锌,即第二代磷酸锌,近年来引起国内外研发人员的高度重视[4-8]。有些第二代磷酸锌产品已经商品化生产,如德国 Heubach 公司生产的水合正磷酸锌铝ZPA(增加了铝离子)、碱式水合磷硅酸锌ZBZ(增加了硅酸根阴离子)、正磷硅酸锌锶钙ZCP(增加了SiO44-,Ca2+和Sr2+);挪威Waardals公司生产的磷硼酸锌WacorZBP-M/263(增加了偏硼酸根);美国Mineral颜料公司的钼酸盐改性的球形超细化磷酸锌J-0806品种。国内对磷酸锌改性研究相关报道较少,本文作者采用价格低廉的阳离子钙取代了部分锌离子水热合成了超细磷酸锌钙防锈颜料,期望通过化学改性获得粒径小于10 μm的超细产品,达到提高颜料的防锈活性及降低成本的目的。水热法是在特制的密闭高压反应釜里,采用水溶液作为反应介质,通过对反应容器加热,创造一个高温、高压反应环境,使得通常难溶或不溶的物质溶解并重结晶。通过对其合成工艺条件的控制,能够达到控制产物的粒度、形貌、相组成、化学均一性等方面的目的[9-10]。本文作者利用水热法制备得到超细CaZn2(PO4)2粉体,主要研究了反应工艺条件对产物粒径、粒径分布、形态等方面的影响,同时探讨了反应机理及产物失水过程。
1 实验
1.1 主要原材料
H3PO4,分析纯,广州市东红化工厂生产;ZnO,化学纯,汕头市光华化学厂生产;Ca(OH)2,化学纯;天津市福晨化学试剂生产; OP-10,分析纯,天津市瑞金特化学品有限公司生产;PEG-600,分析纯,天津市百世化工有限公司生产;PEG-2000,分析纯,天津市大茂化学试剂厂生产。
1.2 超细CaZn2(PO4)2粉体的制备
将ZnO与Ca(OH)2 按一定比例混合,加入适量的表面活性剂与蒸馏水配制成一定量的混合溶液,置于100 mL烧瓶中,用磁力搅拌0.5 h,同时缓慢滴加稀释的H3PO4。制得水热反应前驱物,然后倒入聚四氟乙烯罐,一起放置于反应釜中,在釜中可以加入一定量的蒸馏水,控制一定的填充度,密封升温。反应温度为80~120 ℃,时间小于12 h。反应结束后将高压釜打开,取出聚四氟乙烯罐,将反应物作固液分离,分离之后的沉淀物进行干燥,得到产品。
1.3 测试方法
(1) 采用日本HORIBA公司生产的LB-550型动态光散射粒度分析仪进行粒径分析,测试粒度范围为0.8~6 500 nm。
(2) 采用荷兰Philips公司的XL-30FEG型扫描电子显微镜观察显微形貌。
(3) 采用日本理学公司的D/max-ⅢA型X线全自动衍射仪分析反应机理,扫描范围为10°~80°,扫描速度为0.216°/s。
(4) 采用美国TA公司的SDT2960型TGA-DSC示差扫描量热仪进行热重分析,升温速率20 ℃/min,测试过程在氮气保护下进行。
2 结果与讨论
2.1 反应条件对产品粒径的影响
防腐蚀涂料用的磷酸锌钙颜料的粒径及其分布对防腐蚀涂料性能具有非常重要的影响。如果粒径颗粒过大,不仅使其储藏稳定性变差,在使用过程中会出现堵塞设备等问题,甚至使得涂料的着色强度、光泽性等性能变差。因此需对合成磷酸锌钙的粒径进行详细的分析。
根据前期合成工艺条件对磷酸锌钙的粒径影响所得到的一些规律,选择了合成过程中摩尔比n(Ca):n(Zn)作为因素A,反应温度为因素B,反应时间为因素C,表面活性剂种类为因素D,设计出正交实验表,建立4因素3水平正交实验表L9(34)正交实验,采用动态光散射粒度分析仪测得的CaZn2(PO4)2粉体的粒径,分别研究各因素水平对CaZn2(PO4)2粉体的粒径影响,实验结果及极差分析见表1。
由表1可知:对CaZn2(PO4)2粉体粒径的影响程度从大到小依次为:D1,B1,A1,C1,即表面活性剂对磷酸锌钙平均粒径影响最大,反应温度次之,反应时间影响最小。最佳反应条件是:n(Ca):n(Zn)=1:2,反应温度为80 ℃,反应时间为12 h,OP-10为表面活性剂。非离子表面活性剂OP-10可降低氢氧化钙溶液的表面张力,形成临界胶束,在新成长的磷酸锌钙表面吸附,产生空间位阻效应,阻止了磷酸锌钙粒子的聚附长大,达到控制磷酸锌钙颗粒粒径的目的。在研究的温度范围内,随着温度的升高,颗粒粒径呈增大趋势。溶液温度越高,反应物成核过程越激烈,晶体生成的速率加快,晶体长大速率也加快,所以粒径呈增大趋势,这一结果与施尔畏等[11]的观点一致。
2.2 形貌分析
图1所示为样品的扫描电镜图。从图1可以看出:颗粒粒径及其分布与动态光散射粒度测试结果一致。得到的粒径分布比较均匀,粒径集中在1~2 μm。产物的基本形状为片状,还含有一些块状及不规则的形状。主要是由于得到的产物没有经过清洗、过滤,产物中各种杂质所致。同时也可以很明显地观察到:图1(b)产物分散性及粒径都要比图1(a)的差,并且含有杂质也更多。由此可得出反应浓度比例、表面活性剂种类等条件对产物的形貌有很大影响。
表1 试验数据正交分析结果
Table 1 Orthogonal analysis of test data
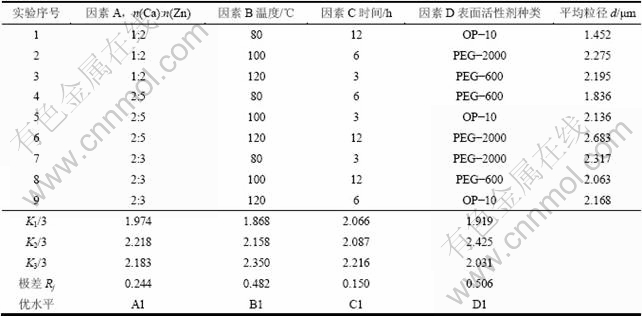
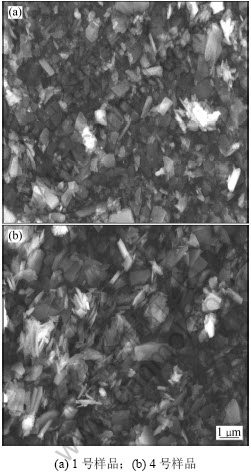
图1 样品的SEM像
Fig.1 SEM images of samples
2.3 反应机理研究
在本次实验过程中,水热反应得到的前驱物及固液分离得到的沉淀物都没有经过洗涤、过滤,保留了合成反应可能得到的各种产物,以便进行反应机理的研究。在正交实验中,1号样品反应条件下,样品的XRD谱如图2所示。与标准衍射卡对照分析可知:制得的产物特征峰以CaZn2(PO4)2·2H2O和Ca[Zn(OH)3]2·2H2O为主,还存在少量没有反应完全的杂质峰。由此可知:最终产物CaZn2(PO4)2·2H2O的形成是通过中间产物Ca[Zn(OH)3]2·2H2O与磷酸反应而生成的。反应过程可用化学式描述如下:
Ca(OH)2+2ZnO+4H2O=Ca[Zn(OH)3]2·2H2O
Ca(Zn(OH)3)2·2H2O+2H3PO4=
CaZn2(PO4)2·2H2O+6H2O
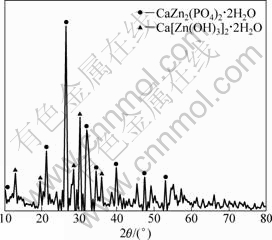
图2 1号样品的XRD图谱
Fig.2 XRD pattern of sample 1
2.4 热重分析
图3所示为正交实验中1号样品的TG曲线。从差热分析结果可以看出:产物的失水过程可以分为2个阶段,第1阶段失水温度从95 ℃开始,到150 ℃基本结束,完全失水结束温度约为393 ℃,第1阶段质量损失约4.0%。第2阶段温度从403 ℃到553 ℃,质量损失为4.98%,可以认为CaZn2(PO4)2·2H2O分子失去另一分子的结合水。因此,2步的质量损失之和8.98%与CaZn2(PO4)2·2H2O分子理论失去2个分子的损失量9%基本近似,故失水过程可以用化学式描述如下:
Ca[Zn(OH)3]2·2H2O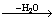 CaZn2(PO4)2·H2O
CaZn2(PO4)2·H2O
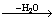 CaZn2(PO4)2
CaZn2(PO4)2
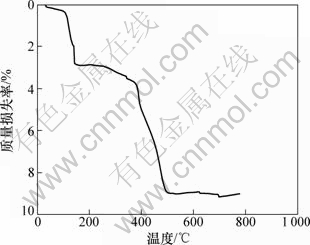
图3 1号样品的TG曲线
Fig.3 TG curve of sample 1
3 结论
(1) 通过设计正交实验确定了水热合成磷酸锌钙粉体的最优方法,并且合成了粒度为1~2 μm的超细CaZn2(PO4)2粉体。合成物虽然存在各种杂质,但合成物粒度均匀。
(2) 水热法合成CaZn2(PO4)2·2H2O过程,首先是ZnO和Ca(OH)2原料反应生成中间产物Ca[Zn(OH)3]2·2H2O,然后Ca[Zn(OH)3]2·2H2O与H3PO4发生反应生成最终产物CaZn2(PO4)2·2H2O。
(3) CaZn2(PO4)2·2H2O分子的失水过程是由2个失水阶段来完成。
参考文献:
[1] HE Wen, YAN Shun-pu, WANG Ying-jun, et al. Biomimetic synthesis of mesoporous zinc phosphate nanoparticles[J]. Journal of Alloys and Compounds, 2009, 477(1/2): 657-660.
[2] Boonchom B, Baitahe R, Kongtaweelert S, et al. Kinetics and thermodynamics of zinc phosphate hydrate synthesized by a simple route in aqueous and acetone media[J]. Ind Eng Chem Res, 2010, 49(8): 3571-3576.
[3] Jung S H, Oh E, Shim D, et al. Sonochemical synthesis of amorphous zinc phosphate nanospheres[J]. Bulletin of the Korean Chemical Society, 2009, 30(10): 2280-2282.
[4] Naderi R, Attar M M. Cathodic disbondment of epoxy coating with zinc aluminum polyphosphate as a modified zinc phosphate anticorrosion pigment[J]. Progress in Organic Coatings, 2010, 69(4): 392-395.
[5] DING Shi-wen, WANG Mao-sheng . Studies on synthesis and mechanism of nano-CaZn2(PO4)2 by chemical precipitation[J]. Dyes and Pigments, 2008, 76: 94-96.
[6] 孙雅博, 吴文伟, 吴昆, 等. 固相反应合成多元微肥缓溶磷酸锌铵及其表征[J]. 无机盐工业, 2005, 37(12): 12-14.
SUN Ya-bo, WU Wen-wei, WU Kun, et al. Synthesis and characteristics of multimicronutrient slow-dissolving fertilizer ammonium zinc phosphate by solid state reaction[J]. Inorganic Salt Industry, 2005, 37(12): 12-14.
[7] QIAN Guan-gren, XU Xia, SUN Wei-ming, et al. Preparation, characterization, and stability of calcium zinc hydrophosphate[J]. Materials Research Bulletin, 2008, 43(12): 3463-3473.
[8] Naderi R, Attar M M. Electrochemical study of protective behavior of organic coating pigmented with zinc aluminum polyphosphate as a modified zinc phosphate at different pigment volume concentrations[J]. Progress in Organic Coatings, 2009, 66(3): 314-320.
[9] Lee J W, Kim J I, Min S H, et al. Highly crystalline lithium-manganese spinel prepared by a hydrothermal process with co-solvent[J]. J Power Sources, 2011, 196(3): 1488-1493.
[10] LEI Wang, YANG Ming, LI Guang-hua, et al. Hydrothermal synthesis and characterization of a new three-dimensional hybrid zinc phosphate [Zn2(HPO4)2(4, 4′-bipy)]·3H2O with neutral porous framework[J]. Journal of Solid State Chemistry, 2006, 179(1): 156-160.
[11] 施尔畏, 夏长泰, 王步国, 等. 水热法的应用与发展[J]. 无机材料学报, 1996, 11(2): 193-206.
SHI Wen-wei, XIA Chang-tai, WANG Bu-guo, et al. Development and application Of hydrothermal method[J]. Journal of Inorganic Materials, 1996, 11(2): 193-206.
(编辑 赵俊)
收稿日期:2011-03-05;修回日期:2011-05-26
基金项目:广东省“211”学科建设项目(412110903-1108)
通信作者:曹有名(1962-),男,湖南宁乡人,教授,博士生导师,从事功能高分子材料、助剂等研究;电话:15902042568;E-mail:youmingcao@sina.com