
Dispersion of copper oxide supported onγ-alumina and its sulfation properties
YU Qing-chun1, ZHANG Shi-chao2, YANG Bin1
1. National Engineering Laboratory of Vacuum Metallurgy, School of Metallurgy and Energy Engineering,
Kunming University of Science and Technology, Kunming 650093, China;
2. School of Materials Science and Engineering, Beihang University, Beijing 100083, China
Received 3 December 2010; accepted 15 September 2011
Abstract: CuO/γ-Al2O3 catalysts were prepared by impregnation with different CuO loadings. The dispersion of CuO supported on γ-Al2O3 support was studied using X-ray diffraction (XRD), scanning electron microscopy (SEM), and temperature programmed reduction (TPR). The dispersion threshold of CuO in γ-Al2O3 determined by X-ray quantitative analysis was 0.275 g/g, i.e., 0.275CuAl. Highly dispersed CuO or crystalline CuO would appear on the γ-Al2O3 support when CuO loading was below or more than its dispersion threshold. TPR experiments show that reduction peak temperature ranges of 0.1CuAl and pure CuO are 420-690 ℃ and 290-380 ℃, respectively. 0.1CuAl is not easily reduced due to interaction between CuO and γ-Al2O3. 0.5CuAl shows a two-step reduction range during 210-300 ℃ and 410-730 ℃, which confirms the existence of highly dispersed CuO and crystalline CuO. The sulfation experiments show the optimal CuO loading amount is far below its dispersion threshold, and copper oxide supported on γ-Al2O3 is in the form of submonolayer.
Key words: copper oxide; dispersion; submonolayer
1 Introduction
Copper oxide has been received considerable attention in recent years for the removal of soot from diesel engine exhaust, the selective oxidation of CO in excess hydrogen, SO2 removal from stack gas [1-4], etc. The active ingredient copper oxide is generally dispersed on porous supports of inert metal oxides such as γ-Al2O3 and SiO2 to augment its catalytic activity. Many metal oxides have a much higher capacity for the formation of atomically dispersed surface species on supports, and the physical and chemical properties of highly dispersed surface species are usually drastically different from those of the corresponding bulk phases. The interaction between copper oxide and support has been of interest in heterogeneous catalysis for many years, not only because of their importance in catalytic applications, but also because of the diversity of explanations of the nature and structure of the dispersed metal oxides on various supports [5-8].
For supported copper oxide catalysts, it has been found that the dispersion state of copper oxide on the support significantly influenced its catalytic properties. YAO et al [9] studied catalytic decomposition of nitrous oxide on CuO/γ-Al2O3 catalysts prepared by grafting, and they found that the properties of these catalysts changed with the amount of deposited metal oxide. HU et al [10] carried out a series work on the dispersion of metal oxides on supports and pointed out that Cu2+ ions occupy the surface octahedral vacant sites of γ-Al2O3 at the pre-impregnation procedure. WANG et al [11] proposed that a great many oxides and salts could disperse spontaneously onto the surfaces of supports to form a monolayer or submonolayer, because in these cases the monolayer was a thermodynamically stable form. The utmost dispersion capacity was called dispersion threshold.
Despite of the studies mentioned above, the effect of the loading amount on activity of copper oxide supported on γ-Al2O3 and desulfurization properties have rarely been reported. In the present work, a series of CuO/γ-Al2O3 catalysts were prepared and the effect of loading amount was studied.
2 Experimental
CuO/γ-Al2O3 sample was prepared by wet impregnation with a known mass of γ-Al2O3 (BET surface area 277.8 m2/g) and solution containing a calculated amount of Cu(NO3)2·3H2O. Followed by evaporation at 90 ℃ with stirring, the samples were dried in an oven at 120 ℃ for 12 h, and subsequently calcined at 450 ℃ in stagnant air for 5 h in a muffle furnace. In this way, samples containing 0.07 g CuO, 0.10 g CuO, 0.12 g CuO, 0.12 g CuO, 0.15 g CuO, 0.35 g CuO, and 0.40 g CuO, 0.45g CuO, 0.55g CuO per gram γ-Al2O3 were obtained, respectively, which were labeled as 0.07 CuAl , 0.10 CuAl, etc.
Temperature-programmed reduction (TPR) experiments were carried out in a thermogravimetric setup with a 5% (volume fraction) H2/Ar mixture and a heating rate of 10 ℃/min. Desulfurization experiments were carried out in the same setup as in TPR experiments. The calculated gas composition was 2×10-3 SO2, 5% O2, and 3% H2O, with N2 as the balance. Chemical reactions occurring during the reduction of CuO by H2 and desulfurization of CuO were as follows:
CuO+H2↑=Cu+H2O↑ (1)
CuO+SO2↑+1/2O2↑=CuSO4 (2)
Due to the consumption of H2, SO2 and O2, and the production of H2O gas in these two reactions, the total mass of sample was changed continuously during reaction. Mass change could be used to evaluate the activity of sample. Mass gain (Δm=mt-m0) plotted as a function of time was obtained from thermogravimetry results, where mt and m0 were actual and initial catalyst masses, respectively. Correspondingly, the mass loss, plotted as a function of temperature, was negative.
X-ray diffraction (XRD) for identification of crystalline phases in the catalysts was performed in a D/MAX diffractometer with Cu Kα radiation (40 kV, 50-100 mA). XRD patterns were recorded in the range 10°≤2θ≤80°. A scanning electron microscope was used to follow the surface morphology.
3 Results and discussion
3.1 XRD analysis of CuO supported on γ-Al2O3
XRD patterns of CuO/γ-Al2O3 sorbents with different copper loadings are shown in Fig. 1. For samples with lower CuO loadings, evident peaks corresponding to the crystalline CuO are not observed in Fig. 1(a) , which implies that the copper oxide species are highly dispersed on the surface of γ-Al2O3 support. When the CuO loading exceeds a critical value, i.e., dispersion threshold, crystalline CuO presents increasing intensity with loading as shown in patters (b), (c), (d), and (e) in Fig. 1.
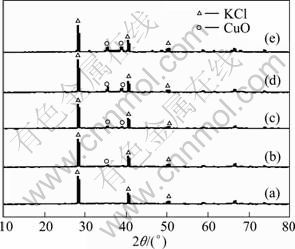
Fig. 1 XRD patterns of CuO/γ-Al2O3 samples with different CuO loadings and reference material KCl: (a) 0.1 CuAl+KCl; (b) 0.35 CuAl +KCl; (c) 0.4 CuAl +KCl; (d) 0.50 CuAl +KCl; (e) 0.55 CuAl +KCl
The dispersion threshold determination was done by X-ray quantitative analysis with internal-standard material KCl [12]. For a binary mixture of compound CuO and matrix γ-Al2O3, the relation between X-ray diffraction intensities and mass fraction of CuO is shown in Fig. 2.
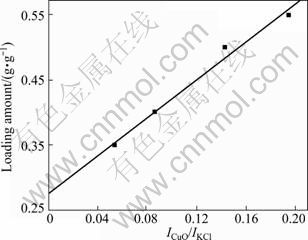
Fig. 2 Diffraction intensity ratio of CuO to KCl versus CuO loading amount
The intercept in Fig. 2 is the dispersion threshold of 0.275CuAl. As metal loading increases, X-ray diffraction of CuO appears, and microparticles of CuO form on the γ-Al2O3 support. The theoretical dispersion value of CuO is 0.529 according to O2- anion radius of 0.132 nm, which means that CuO is dispersed on γ-Al2O3 in the form of submonolayer.
3.2 Scanning electron microscopy (SEM) analysis
In order to examine the dispersion of CuO on γ-Al2O3 support, stereographic pictures of 0.1CuAl and 0.5CuAl taken from a scanning electron microscope are shown in Fig. 3. The even surface shown in Fig. 3(a) means that copper oxide supported on γ-Al2O3 is highly dispersed. On the other hand, the higher CuO loaded sample 0.5CuAl shows clearly differentiated nano-CuO in Fig. 3(b). Dispersion states of CuO with loading amount below and above its dispersion threshold coincide with the XRD results above.
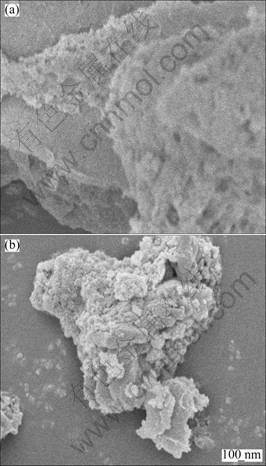
Fig. 3 SEM images of 0.1CuAl (a) and 0.5CuAl (b)
3.3 TPR analysis
The reduction characteristics of the prepared samples were studied over the temperature range of 100-900 ℃ by temperature-programmed-reduction(TPR) given in Fig. 4.
Mass loss of pure CuO in the temperature range of 290-380 ℃ shows that unsupported CuO is easily reduced by H2. A gentle mass loss of 0.1CuAl in the temperature range of 420-690 ℃ shows that reduction of copper oxide is difficult due to its interaction with the support as CuO loading amount is below its dispersion threshold. The mass loss of 0.1CuAl approximates to the theoretical value following Eq. (1), which means that the reduction product is Cu. X-ray diffraction measurements for the residual products were conducted, and no evidence was obtained for the presence of Cu2O. However, the mass loss of 0.5CuAl occurs in a two-step temperature range of 210-300 ℃ and 410-730 ℃, respectively, which shows that there are two reduction processes. The first one denotes the reduction of crystallite CuO on the support. The lower reduction temperature of crystalline CuO means that nano-CuO is more active than normal size CuO. The higher one denotes the reduction of amorphous CuO on γ-Al2O3 support, which coincides with the reduction of 0.1CuAl. Above dispersion threshold, copper oxide would exist in the form of highly dispersed and crystalline states on γ-Al2O3 support, and CuO in crystalline state can be detected by XRD, such as 0.5CuAl shown in Fig. 1.
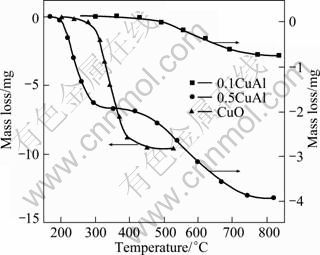
Fig. 4 TPR profiles of 0.1CuAl, 0.5CuAl and CuO
3.4 Sulfation experiment
In order to understand the catalytic oxidation of copper oxide supported on γ-Al2O3, sulfation of CuO/γ-Al2O3 with different CuO loading amounts is performed. Mass gain caused by the reaction of CuO/γ-Al2O3 sorbents with SO2 at 350 ℃ as a function of time is shown in Fig. 5(a). With increasing CuO loading amount, the mass gain should increase until CuO loading amount reaches its dispersion threshold. However, the specific rate of sulfation passes through a maximum for a defined surface coverage of copper oxide on alumina, i.e., 0.12 g CuO per gram γ-Al2O3 shown in Fig. 5(b), much lower than its dispersion threshold.
From a microscopic point, metal oxide surfaces are mainly composed of oxygen atoms and hydroxyl groups. According to the close-packed monolayer model [11], a great many oxides and salts can disperse spontaneously onto the surfaces of supports to form a monolayer or submonolayer. This model is on the assumption that O2- anions form a close-packed monolayer, and metal cation occupies the vacant between O2- anions. The schematic diagram is shown in Fig. 6.
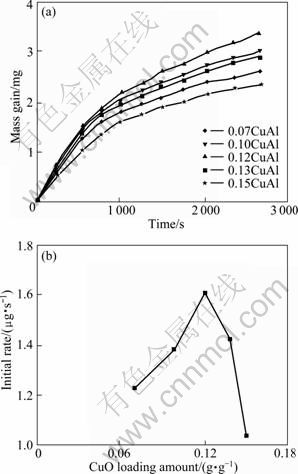
Fig. 5 Sulfation of CuO/γ-Al2O3 with different CuO loading amounts: (a) Effect of CuO loading amount versus time; (b) Initial rate versus CuO loading amount
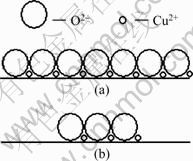
Fig. 6 Schematic diagram of close-packed model: (a) Monolayer; (b) Submonolayer
The dispersion threshold gave a meaningful explanation of highly dispersed oxides and crystalline oxides from thermodynamic point. If all the surface of γ-Al2O3 support is covered with CuO, γ-Al2O3 support will not participate in catalytic reaction. However, further study showed that the surface of γ-Al2O3 support participated in sulfation reaction under condition of a long sulfation time [13]. The amount introduced onto the porous support depends on the equilibrium concentration of the impregnating solution, the porous volume of the γ-Al2O3 support and the adsorption isotherm which describes the binding of the precursor onto the support surface [14]. Considering these factors, it seems impossible that γ-Al2O3 is covered by CuO thoroughly uniformly. CuO in the form of submonolayer is more reasonable.
TWU [15] reported three structures about surface of sulfates as shown in Fig. 7, and one sulfur atom lines two or three oxygen atoms. When a CuO molecule is transformed into CuSO4, the SO42- ionic group may cover the adjacent CuO molecules, which may cause the optimal CuO loading amount far below its theoretical dispersion threshold.
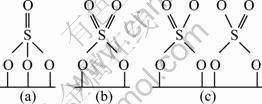
Fig. 7 Structure diagram of sulfates
4 Conclusions
1) The dispersion threshold of CuO supported on γ-Al2O3 support is 0.275CuAl. Scanning electronic micrographs show that highly dispersed CuO or crystalline CuO will exist on the γ-Al2O3 support when CuO loading amount is below or above its dispersion threshold.
2) Reduction peak temperature ranges of 0.1CuAl and pure CuO are 420-690 ℃ and 290-380 ℃, respectively, which means that 0.1CuAl is not easily reduced due to the interaction between CuO and γ-Al2O3. 0.5CuAl shows a two-step reduction range of 210-300 ℃ and 410-730 ℃, which confirms the existence of highly dispersed CuO and crystalline CuO. The lower reduction temperature of crystalline CuO denotes that nano-CuO is more active than normal size CuO.
3) Catalytic oxidation experiments show that the optimal CuO loading amount is 0.12CuAl, which is much lower than its dispersion threshold.
References
[1] LOPEZ-SUAREZ F E, BUENO-LOPEZ A, ILLAN-GOMEZ M J. Cu/Al2O3 catalysts for soot oxidation: Copper loading effect [J]. Applied Catalysis B: Environmental, 2008, 84: 651-658.
[2] PARK J W, JEONG J H, YOON W L, JUNG H, LEE H T, LEE D K, PARK Y K, RHEE Y W. Activity and characterization of the Co-promoted CuO–CeO2/γ-Al2O3 catalyst for the selective oxidation of CO in excess hydrogen [J]. Applied Catalysis A: General, 2004, 274: 25-32.
[3] YONG M, HUBERT G, RALF Z, VOLKER H, CHRISTIANE Z, GUNTHER K. Steam reforming of methanol over Cu/CeO2/γ-Al2O3 catalysts in a microchannel reactor [J]. Applied Catalysis A: General, 2004, 277: 83-90.
[4] WAQIF M, SAUR O, LAVALLEY J C, PERATHONER S, CENTI G. Nature and mechanism of formation of sulfate species on copper/alumina sorbent-catalyst for SO2 removal [J]. Journal of Physical Chemistry, 1991, 95: 4051-4058.
[5] GAO Yang, ZHAO Hai-bo, ZHAO Bi-ying. Monolayer dispersion of oxide additives on SnO2 and their promoting effects on thermal stability of SnO2 ultrafine particles [J]. Journal of Material Science, 2000, 35: 917-923.
[6] CHEN Kai-dong, DONG Lin, YAN Qi-jie, CHEN Yi. Dispersion of Fe2O3 supported on metal oxides studied by Mossbauer spectroscopy and XRD [J]. Journal of Chemistry Society Faraday Transaction, 1997, 93(12): 2203-2206.
[7] SALAGRE P, FIERRO J L G, MEDINA F, SUEIRAS J E. Characterization of nickel species on several γ-alumina supported nickel samples [J]. Journal of Molecular Catalysis A: Chemical, 1996, 106: 125-134.
[8] HAO Xiang-ying, ZHANG Cui, ZHANG Yin-qing, WANG Zhen-yu, LIU Shuang-xi. Study on the dispersion of CuO on the MCM-41 surface [J]. Journal of Molecular Catalysis (China), 2006, 20(1): 73-75. (in Chinese)
[9] YAO K W, JAENICKE S, LIN J Y, TAN K L. Catalytic decomposition of nitrous oxide on grafted CuO/γ-Al2O3 catalysts [J]. Applied Catalysis B: Environmental, 1998, 16: 291-301.
[10] HU Yu-hai, WANG Jun, DING Wei-ping, CHEN Yi. Activities of supported copper oxide catalysts in the NO+CO reaction at low temperatures [J]. Journal of Molecular Catalysis A: Chemical, 2000, 162: 307-316.
[11] WANG Chun-ming, ZHAO Bi-yi, XIE You-chang. Advances in the studies of spontaneous monolayer dispersion of oxides and salts on supports [J]. Chinese Journal of Catalysis, 2003, 24(6): 475-482.
[12] MIAO Chun-sheng. X-ray quantitative analysis methods [M]. Beijing: Geological Press, 1988: 3-10. (in Chinese)
[13] YU Qing-chun, ZHANG Shi-chao, WANG Xin-dong. Thermogravimetric study of CuO/γ-Al2O3 sorbents for SO2 in simulated flue gas [J]. Industrial Engineering Chemistry Research, 2007, 46: 1975-1980.
[14] SCHWARZ J A, CONTESCU C, CONTESCU A. Methods for preparation of catalytic materials [J]. Chemistry Review, 1995, 95: 477-510.
[15] TWU J, CHUANG C J, CHANG K I, YANG C H, CHEN K H. Raman spectroscopic studies on the sulfation of cerium oxide [J]. Applied Catalysis B: Environmental, 1997, 12: 309-324.
氧化铜在γ-Al2O3载体表面的分布及其硫化性能
郁青春1, 张世超2, 杨 斌1
1.昆明理工大学 冶金与能源工程学院,真空冶金国家工程实验室,昆明 650093;
2. 北京航空航天大学 材料科学与工程学院,北京 100083
摘 要:采用浸渍方法制备不同氧化铜含量的CuO/γ-Al2O3催化剂。使用XRD、SEM和程序控制升温还原(TPR)方法研究γ-Al2O3载体表面氧化铜的分布情况。用X射线定量分析方法得到氧化铜在γ-Al2O3中的分散阈值为0.275 g/g,即 0.275CuAl。当氧化铜负载量小于或大于其分散阈值时,高度分散或结晶状氧化铜将会出现在γ-Al2O3载体表面。TPR实验表明0.1CuAl 和纯 CuO还原温度范围分别为420-690 ℃和290-380 ℃。氧化铜与载体之间的相互作用导致0.1CuAl不易被还原。 0.5CuAl呈现2个分步还原温度范围,分别为210-300 ℃ 和410-730 ℃,证实了γ-Al2O3载体表面高度分散和结晶状分布的氧化铜。硫化实验表明,最佳氧化铜负载量小于其分散阈值,氧化铜以亚单层形式分布在γ-Al2O3载体表面。
关键词:氧化铜;分散;亚单层
(Edited by LI Xiang-qun)
Foundation item: Project (Jinchuan 201114) supported by the Pre-Research Foundation of Jinchuan Group Ltd., China; Project (2011148) supported by the Analysis and Testing Foundation of Kunming University of Science and Technology, China
Corresponding author: YU Qing-chun; Tel: +86-871-5161583; E-mail: ygcy@163.com
DOI: 10.1016/S1003-6326(11)61104-7