
Influence of multilayer coatings on AZ31 deposited by
electron beam evaporation
WANG Xue-min(王雪敏)1, ZENG Xiao-qin(曾小勤)1, YAO Shou-shan(姚寿山)1, LAI Yi-jian(赖依建)2
1. National Engineering Research Center of Light Alloys Net Forming, Shanghai Jiao Tong University,
Shanghai 200030, China;
2. Instrumental Analysis Center, Shanghai Jiao Tong University, Shanghai 200030, China
Received 28 July 2006; accepted 15 September 2006
Abstract: Three different coatings, Cr, SiO2/Cr and HfO2/SiO2 were deposited by electron beam physical vapor deposition (EB-PVD). Relevant composition and mechanical properties were obtained. The results show that the surface mechanical property could be increased, and Cr coating sample possessed the highest microhardness. Cyclic oxidation in air at 773 K was applied to evaluate the oxidation resistance of the coatings, and the section morphologies of the coatings were observed by FEISEM. The results indicate that the oxidation rate of AZ31 with Cr, SiO2/Cr and HfO2/SiO2 coatings is decreased, and the SiO2/Cr coating sample exhibits the best oxidation resistance and keeps relatively good adhesion up to 96 h. Polarization results prove that the corrosion resistance of AZ31 can be improved and the SiO2/Cr coating sample has the best property.
Key words: magnesium alloy; physical vapor evaporation; cyclic oxidation; corrosion resistance
1 Introduction
Magnesium alloys exhibit low cost, large availability and the lowest density among the engineering metallic materials[1-2]. Due to such excellent properties, magnesium alloys are being increasingly applied in the aerospace, electronic and automotive industries. However, because of their poor corrosion resistance the application of magnesium alloys has been greatly limited. One of the most effective ways to prevent corrosion is to coat magnesium alloys. Surface modifications such as electrochemical plating, chemical vapor deposition (CVD), physical vapor deposition (PVD) and laser surface cladding seem to be a possibility to overcome these drawbacks[3-7]. Among these techniques, electron beam physical vapor deposition (EB-PVD) is an advanced method of ceramic deposition process[8]. Due to its high deposition rate and simplification, EB-PVD has been developed to prepare the corrosion protective coatings for industrial applications[9].
Most previous work conducted by PVD method on the surface properties of magnesium alloys have been concentrated on ceramic coatings such as Al2O3/Al, Al2O3/Ti, TiN, CrN, TiAlN, NbN-(TiAl)N, CrN-TiCN and multi-layer composite AlN/TiN[10-12]. In this study, Cr, SiO2/Cr and HfO2/SiO2 coatings were deposited by EB-PVD. The corresponding corrosion resistance and oxidation behavior were investigated.
2 Experimental
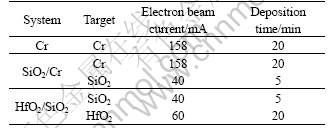
Rectangular coupons (10 mm×10 mm×3 mm) of die cast AZ31 magnesium alloys were applied as the samples. AZ31 magnesium alloy is an alloy containing(in mass fraction): 2.5-3.5% Al, 0.5-1.5%Zn and Mg balance. Prior to deposition process, all samples were mechanically polished with 800 grid SiC paper, and then ultrasonically rinsed in acetone. High-purity Cr pellets (99.99%), HfO2 pellets (99.99%) and SiO2 pellets (99.99%) were used as the sources. In all cases, the initial vacuum level of the target chamber was kept at 1×10-3 Pa, and the acceleration voltage was 6 kV. The coating time was controlled to obtain a thickness of 2 to 3 μm. Table 1 summarizes the corresponding coating conditions.
X-ray diffraction (BRUKER AXS D8 advance diffractometer) with Cu Kα(λ=0.154 056 nm) radiation was used to identify the composition of the coatings. Cyclic oxidation test was performed in a box-type furnace. For each cycle, the samples were exposed in air at 773 K for 4 h and then cooled to room temperature for 15 min to measure the mass gain. Scanning electron microscopy (FEI, SIRION-200) was employed to observe the section morphologies of the coatings after cyclic oxidation test. The potentiodynamic tests were performed in a 3.5%NaCl solution saturated with Mg(OH)2 using a Parstat 2273 potentiostat system. A three electrode cell with the sample as the working electrode, Ag/AgCl/KCl(sat) electrode as the reference electrode, and a platinum rod as the counter electrode was used in the tests. The tested area was 1 cm2, and the scan rate was 0.166 mV/s.
Table 1 Parameters in deposition process
3 Results and discussion
3.1 Composition of coatings
All coatings were investigated by XRD (Fig.1). Due to the thin thickness of the coatings, diffraction peaks of the substrate were obviously observed. As shown in Fig.1, it can be found that the diffraction peaks of the single Cr coating are weak, but seem to change relatively stronger for the double-layer SiO2/Cr coating. However, it is obvious that the diffraction peaks of the HfO2/SiO2 coating exhibits a different pattern and shows a broad peak. This fact suggests that the HfO2/SiO2 coating possesses a amorphous structure.
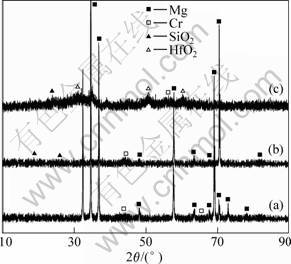
Fig.1 XRD patterns of coatings: (a) Cr coating; (b) SiO2/Cr coating; (c) HfO2/SiO2 coating
3.2 Surface mechanical properties
The microhardness of bare AZ31 and coated AZ31 samples at different loads is presented in Fig.2. In this study, four types of loads (490, 980, 1 960 and 2 940 N) were applied respectively, and kept loading on the surface of the samples up to 15 s. It was unexpected that the hardness of Cr coating was higher than that of SiO2/Cr and HfO2/SiO2 coatings. It may be due to the reason that double-layer coatings were amorphous structure, which was confirmed by the X-ray diffraction results (Section 3.1). Moreover, with the increasing loads, the microhardness of the coatings was gradually close to the substrate. This phenomenon indicates that up to high loads the load was mainly supported by the substrate. Considered other coating’s physical properties, the surface mechanical property may be affected by hardness, thickness, structure and adhesion[13].
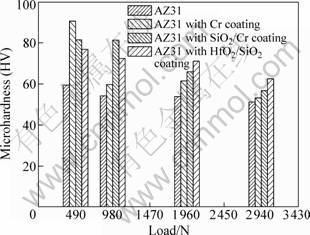
Fig.2 Microhardness of samples at different loads
3.3 Oxidation kinetics
Cyclic oxidation tests were performed in air at 773 K to evaluate the mass gain—time curves of the samples. The results are shown in Fig.3. As indicated in this figure, the bare AZ31 exhibits a linear increase in mass, in agreement with the results of ZHANG[14]. The continuous oxidation of AZ31 can be ascribed to the porous structure of MgO film[15]. As a result of this porous structure, the oxide film can not act as a barrier to prevent the inward diffusion of oxygen and magnesium can be oxidized continuously. Two stages can be distinguished in the mass—gain curve of the Cr coating sample. The first stage obeys a parabolic behavior, while the second stage fits quasi-linear kinetics. Such a transition suggests that Cr coating can protect the substrate from oxidation only for the initial 40 h. The mass gain curve of the SiO2/Cr coating sample is similar to that of the HfO2/SiO2 coating sample. After about initial 40 h cyclic oxidation, the mass decreased gradually. This phenomenon shows that the coatings become to spall after longer cyclic oxidation. On the other hand, the gradual and stable decrease in mass suggests a relatively good adhesion of the coatings[16]. Thus, from Fig.3, it can be concluded that the HfO2/SiO2 coating has the better oxidation resistance than the SiO2/Cr coating against cyclic oxidation.
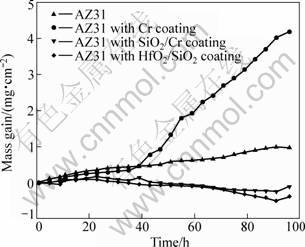
Fig.3 Mass—gain curves of samples in air at 773 K
Fig.4 shows the macrographs of the samples after cyclic oxidation in air at 773 K for 96 h. The surface of the bare AZ31 sample was covered with a lot of gray oxide, mainly consisted of MgO according to EDX measurement. Cracked and spalled surface was observed on both Cr coating and HfO2/SiO2 coating, and some local places of the substrate were exposed to the environment. The Cr coating seemed to be severe oxidized, in agreement with the results in Fig.3. Only a little spalled area could be found on the SiO2/Cr coating and the surface kept wholly. So, it can be concluded that the SiO2/Cr coating exhibits the best oxidation resistance.
Fig.5 shows the section morphologies observed by SEM. In agreement with the mass gain (Fig.3), the Cr coating was completely cracked and spalled. A lot of mushroom oxidation scale was produced on the surface, which might result from the volume expansion of magnesium oxidation on grain boundaries. Compared with the Cr coating, the HfO2/SiO2 coating exhibited a low density of cracks and spallation. However, with oxidation process the coating could not inhibit the further oxidation of the substrate. According to Fig.5 (b), it seemed that no obvious cracks and serious spallation existed in the SiO2/Cr coating, and only some small pores were found. So, the SiO2/Cr coating sample was concluded to have the best oxidation resistance.

Fig.4 Surface macrographs of samples after oxidation at 773 K for 96 h: (a) AZ31; (b) Cr coating; (c) SiO2/Cr coating; (d) HfO2/SiO2 coating
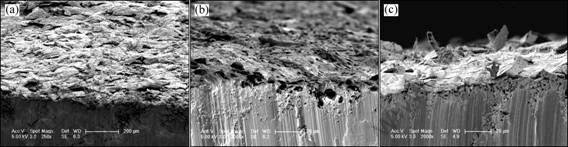
Fig.5 Cross section morphologies of coated samples after cyclic oxidation in air at 773 K for 96 h: (a) Cr coating; (b) SiO2/Cr coating; (c) HfO2/SiO2 coating
Summarizing, the present study proves that the SiO2/Cr coating possesses the best oxidation resistance. Moreover, by decreasing the density of defects generated during PVD synthesis the oxidation resistance can be further improved.
3.4 Corrosion resistance
Potentiodynamic polarization was performed in order to evaluate the corrosion resistance of the coating samples. Fig.6 shows the potentiodynamic polarization curves, and the corresponding polarization data are summarized in Table 2. Ecorr is the potential at which the current changed polarities. As indicated in Fig.6 and Table 2, it was found that the corrosion potential of the Cr coating and the HfO2/SiO2 coating shifted positively compared with the bare sample, while the corrosion current density of the SiO2/Cr coating sample was decreased. Therefore, this fact demonstrates that by prepared the SiO2/Cr coating the corrosion resistance of AZ31 magnesium alloys can be improved. The result is consistent with the conclusion derived from the cyclic oxidation test (Section 3.3).

Fig.6 Potentiodynamic polarization curves exposed to 3.5% NaCl solution saturated with Mg(OH)2
Table 2 Polarization data

As reported in Refs.[17-20], to improve the oxidation resistance of materials by coatings the deposited film needs to be low porous and has good adhesion to the surface. Thus, the dense coatings can reduce the inward diffusion of oxygen and the corresponding oxidation rate is decreased. On the other hand, it is well known that pitting corrosion is the main corrosion form in Cl- containing solution for magnesium alloys[21-22]. To enhance the corrosion resistance of magnesium alloys, the coatings should inhibit the transfer of Cl-. Therefore, the oxidation properties and the corrosion resistance suggest that the SiO2/Cr coating has better adhesion and low porous compared with other coatings.
4 Conclusions
1) Three different coatings, Cr, SiO2/Cr and HfO2 /SiO2 were deposited on AZ31 magnesium alloys by electron beam physical vapor deposition.
2) Cyclic oxidation test indicates that the SiO2/Cr coating sample possesses the best oxidation resistance against cyclic thermal stress and even after 96 h it still remains protective.
3) Potentiodynamic polarization results show that the SiO2/Cr coating can improve the corrosion resistance of AZ31 magnesium alloys.
References
[1] KAINER K U, VON BUCH F. Modern development of alloys for light weight components [J]. Materialwissenschaft and Werks- tofftechnik, 1999, 30(3): 159-167.
[2] GRAY J E, LUAN B. Protective coatings on magnesium and its alloys—A critical review [J]. J Alloys Compd, 2002, 336(1/2): 88-113.
[3] ZHANG R F, SHAN D Y, HAN E H, GUO S B. Development of microarc oxidation process to improve corrosion resistance on AZ91HP magnesium alloy[J]. Trans Nonferrous Met Soc China, 2006, 16(S): 685-688.
[4] LUO J L, CUI N. Effects of microencapsulation on the electrode behavior of Mg2Ni-based hydrogen storage alloy in alkaline solution[J]. J Alloys Comp, 1998, 264(1/2): 299-305.
[5] REINERS G, GRIEPENTROG M. Hard coatings on magnesium alloys by sputter deposition using a pulsed d.c. bias voltage[J]. Sur Coat Tech, 1995, 76/77(1/3): 809-814.
[6] CHEN C J, WANG D S, WANG M C. Laser surface cladding of ZM5 Mg-base alloy with Al+Y powder[J]. Trans Nonferrous Met Soc China, 2004, 14(6): 1091-1094.
[7] CHRISTOGLOU C H, VOUDOURIS N, ANGELOPOULOS G N, PANT M, DAHL W. Deposition of aluminium on magnesium by a CVD process[J]. Sur Coat Tech, 2004, 184(2/3): 149-155.
[8] REINHOLD E, RICHTER J, SEYFERT U, STEUER C. Metal strip coating by electron beam PVD-industrial requirements and customized solutions[J]. Sur Coat Tech, 2004, 188/189(s1/s3): 708-713.
[9] JASCH G, SENF A. Physical vapor deposition on metal strips[C]// PAULA M. Proc Conf EB Melt Englewood[C]. NJ: Ref State of the Art, Reno, Bakish Materials Corp., 1995: 98-100.
[10] HOCHE H, BIAWERT C, BROSZEIT E, BERGER C. Galvanic corrosion properties of differently PVD-treated magnesium die cast alloy AZ91[J]. Sur Coat Tech, 2005, 193(s1/s3): 223-229.
[11] HOCHE H, SCHEERER H, PROBST D, BROSZEIT E, BERGER C. Development of a plasma surface treatment for magnesium alloys to ensure sufficient wear and corrosion resistance[J]. Sur Coat Tech, 2003, 174/175: 1018-1023.
[12] LEHAN J P, MAO Y, BOVARD B G, MACLEOD H A. Optical and microstructural properties of hafnium dioxide thin films[J]. Thin Solid Films, 1991, 203(2): 227-250.
[13] HOLLSTEIN F, WIEDEMANN R, SCHOLZ J. Characteristics of PVD-coatings on AZ31hp magnesium alloys[J]. Sur Coat Tech, 2003, 162(2/3): 261-268.
[14] ZHANG W Q. Handbook of Metal Corrosion[M]. Shanghai: Shanghai Science and Technology Press, 1987.
[15] YOU B S, PARK W W, CHUNG I S. Effect of calcium additions on the oxidation behavior in magnesium alloys[J]. Scripta Materials, 2000, 42(11): 1089-1094.
[16] JUNG H G, WEE D M, Oh M H, KIM K Y. An Al+Y coating process for improvement of the high-temperature oxidation resistance of a TiAl alloy[J]. Oxidation of Metals, 2001, 55(3/4): 189-208.
[17] LEE D B, JANG Y D, MYUNG H S, HAN J G. High-temperature oxidation of magnetron-sputtered Cr-N-coated steels[J]. Thin Solid Films, 2006, 506/507(26): 369-372.
[18] BARDI U, CHENAKIN S P, GHEZZI F, GIOLI C, GORUPPA A, LAVACCHI A, MIORIN E, PAGURA C, TOLSTOGOUZOV A. High-temperature oxidation of CrN/AlN multilayer coatings[J]. Appl Surf Sci, 2005, 252(5): 1339-1349.
[19] MA W, GONG S, XU H, CAO X. The thermal cycling behavior of Lanthanum-Cerium Oxide thermal barrier coating prepared by EB-PVD[J]. Sur Coat Tech, 2006, 200(16/17): 5113-5118.
[20] GUO H F, AN M Z. Growth of ceramic coatings on AZ91D magnesium alloys by micro-arc oxidation in aluminate-fluoride solutions and evaluation of corrosion resistance[J]. Appl Surf Sci, 2005, 246(1/3): 229-238.
[21] CHEN M, YU Q S, REDDY C M, YASUDA H K. Model study investigating the role of interfacial factors in electrochemical impedance spectroscopy measurements[J]. Corrosion, 2000, 56(7): 709-721.
[22] INOUE H, SUGAHARA K, YAMAMOTO A, TSUBAKINO H. Corrosion rate of magnesium and its alloys in buffered chloride solutions[J]. Corrosion Science, 2002, 44 (3): 603-610.
(Edited by YANG Hua)
Corresponding author: WANG Xue-min; Tel: +86-21-62933463; E-mail: wangxuemin75@sohu.com