Article ID: 1003-6326(2005)06-1199-07
Biomimetic coating of calcium phosphate on
biometallic materials
ZHANG Er-lin(张二林), YANG Ke(杨 柯)
(Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China)
Abstract: The biomimetic coating process in comparison with other processes is reviewed. This processing shows advantages in the surface bio-modification, such as low cost and flexible processing, wide range of apatite composition and thickness, non-line-of-sight characteristic and possibility to coat polymers and porous implants. The bio-mimetic apatite coating is made up of larger number of globules with size of 1-5μm. Each globule is a group of numerous flakes with a size range of 100-200nm to 30μm in length and 0.1-1μm in thickness. In-vitro and in-vivo studies show that the biomimetic apatite coating can promote an early and strong bonding to bone or promote the bone in-growth into the porous structure, which will be beneficial to the cementless stable fixation of orthopaedic implants. Recently developed co-precipitation of a kind of protein molecules into the HA coating shows much promising.
Key words: hydroxyapatite coating; biomedical application; biomimetic coating; biometallic materials; surface modification CLC
number: TB39 Document code: A
1 INTRODUCTION
In the recent years, there is a huge development in biomaterials that are specially designed to repair and reconstruct damaged or diseased parts of the human bones[1-5]. Biometallic materials, such as titanium and titanium alloys, have been mainly used as bone implants due to their good biocompatibility. However, a fibrous tissue was normally detected at the interface between bone and bio-material due to its poor osteoconductivity, which finally would result in the lossening of the bone implant. On the other hand, calcium phosphate, especially hydroxyapatite, Ca10(PO4)6(OH)2 or HA, has demonstrated good biocompatibility and osteoconductivity as well as excellent wear properties. However, a component made of solely HA was found to be lack of toughness and tensile strength, and can fail catastrophically. As a result, HA coated titanium components, which combine the high mechanical strength of titanium and the bioactivity of HA, are developed and reckoned to be one of the most promising groups of implant materials in orthopaedic and dental fields.
Many processes have been developed to coat HA onto the titanium substrate. The most widely used coating technique is plasma spraying[6-8], which has been used as a commercial processing technique. However, the plasma-spraying technique presents several drawbacks. Many researchers focus on developing a low cost, high efficiency and low temperature process, such as electrophoretic deposition[9, 10], biomimetic[11-13] and electrochemical deposition[14-17]. In pervious paper, we have reviewed the application of electrophoretic deposition in HA coating[18]. In this paper, we will discuss the application of biomimetic deposition in the bone implant surface modification.
2 BIOMIMETIC COATING PROCESS AND ADVANTAGES
2.1 Biomimetic coating process
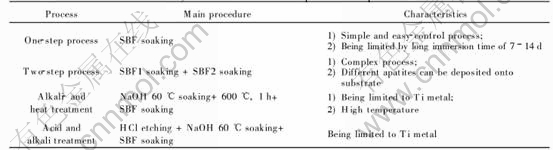
The biomimetic coating process was developed on the base of the heterogeneous nucleation of Ca-P from simulated body fluids(SBF), which has a similar inorganic content as human blood plasma (HBP). Based on the above mechanism, many processes have been developed. In a so-called one-step process[19], implant materials, such as titanium implants, were soaked directly into a buffered SBF solution (pH=7.4) at 37℃. However, the development of such biomimetic Ca-P coatings was limited by long immersion periods of about 7-14d with daily refreshments of SBF solution.
Kokubo et al[20-25] developed an alkali- and heat treatment process. A bonelike apatite layer was formed on the surface of pure titanium metal and Ti-6Al-4V in SBF within 3d after being treated by soaking in 5mol/L NaOH solution at 60℃ for 24h with subsequent heat treatment at 600℃ for 1h. Kato et al[26] has applied this process to tantalum, but it took 7d to deposit Ca-P onto the surface. Some researchers ever have successfully applied this technology to porous metal implants[27].
Further research indicated that the acid etching provides a higher precipitation rate of apatite onto the surface of titanium[28, 29]. In addition, high temperature and high pressure were also applied to the alkaline treatment in order to promote the deposition of apatite on the substrate from SBF[30].
In order to reduce the soaking time and enhance the deposition of apatite-like layers, a so-called two-step process has been developed[11, 31-33]. In this process, implant materials were first soaked into a supersaturated Ca-P solution with high concentrations of salts (SBF1 in Table 1) in order to pre-treat the substrate with Ca-P nuclei. CO2 gas was evolved through the solution in order to increase the pH value and induce the deposition of a thin and amorphous calcium phosphate(ACP) coating on the titanium samples. Then, the implants were immersed into a more concentrated SBF solution (SBF2 in Table 1) in order to grow the bio-mimetic coating. It was reported that uniform and well-attached Ca-P coatings could be deposited on titanium implants within 5h[34], much shorter than that in the former processes. By changing the composition of the SBF2, different calcium phosphate apatites can be deposited onto the substrate, such as carbonate apatite coating(CA) and OCP coating[32]. In addition, the phase composition, the crystallinity and the adhesion of biomimetic Ca-P coatings on titanium were strongly related to the ionic strength, carbonate and magnesium contents in the SBF1 solution.
Table 2 summarises several methods which have been applied to the biomimetic deposition of Ca-P coating in literatures.
One of the new developments in the biomimetic calcium phosphate coating is co-precipitating a kind of protein molecules, such as bovine serum albumin(BSA), with the inorganic components and then they become incorporated into the crystal lattice[13, 35]. This facility means that biomimetically prepared matrices can be used as carriers for organic molecules with bone-healing properties (such as bone morphogenetic proteins or growth factors).
Table 1 Inorganic composition concentration (mmol/L) of HBP, supersaturated Ca-P solution and SBF[32]

Table 2 Summary of several biomimetic processes reported in literatures
These protein molecules are released gradually from these coatings rather than in a single rapid burst, which renders biomimetically prepared coatings as slowly drug-released systems do.
2.2 Advantages of biomimetic coating
1) One of the great advantages of this technique is that it imitates the mode, in which hydroxyapatite bone crystals are formed in the body. The coatings thereby generated are composed of small crystal units, which are more readily degraded by osteoclasts.
2) Another major advantage is that protein molecules can be co-precipitated with the inorganic components and thereby become incorporated into the crystal lattice rather than being merely deposited on the surface. The protein molecules are released gradually from these coatings rather than in a single rapid burst.
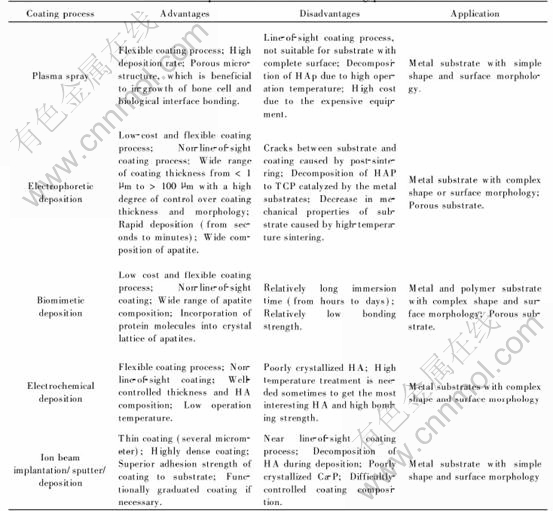
3) The third advantage is that it can be used to deposit even coatings on heat-sensible substrates, such as polymer, and to cover complex shaped materials, such as porous implants, due to its non-line-of-sight and low temperature characteristics.
4) Furthermore, this technique allows the covering of implants with new Ca-P phases that could not be produced at high temperature, such as carbonate apatite(CA) and octacalcium phosphate(OCP). These coatings have different structures and dissolution behaviours.
Table 3 compares the advantages and disadvantages of different coating processes. However, it must be pointed out that the bonding strength of the biomimetic HA coating to the substrate is still lower than that prepared by other processes, such as plasma spray. Further research has to be done to improve the bonding strength.
Table 3 Comparison of several Ca-P coating processes
3 MICROSTRUCTURE AND BIOLOGICAL PERFORMANCE OF BIOMIMETIC APATITE COATING
The biomimetically deposited calcium phosphate film is made up of larger number of globules, with size of 1-5μm, fused together[12, 19]. Each globule is a group of numerous flakes uniting and /or clustering together. The size of the flakes is in the range of 100-200nm to 30μm in length and 0.1-1μm in thickness depending on the processes, as shown in Fig.1 and Fig.2. The molar ratio of Ca to P was estimated to be (1.44±0.09), lower than the stoichiometric 1.67 of hydroxyapatite. XRD results suggested that the apatite was crystallized but the crystals were very small. Some results showed that the Ca-P crystals have grown on the substrate with the favourite orientation of  and [002] while Ca-P crystals were all perpendicularly oriented to the substrate (columnar crystal).
and [002] while Ca-P crystals were all perpendicularly oriented to the substrate (columnar crystal).

Fig.1 SEM micrographs of Ca-P obtained by one-step process at different magnifications[19]

Fig.2 Morphology of Ca-P coating produced by two-step process after 48h soaking[32]
Being one of the main advantages mentioned above, the biomimetic coating can be used to deposit Ca-P coating on substrates with complex shape or surface morphology due to the non-line-of-sight, such as porous substrate[36-38]. Du et al[36] deposited apatite coating on the porous polyactive 1000 PEGT70PBT30 implants by use of the biomimetic coating process. A uniform apatite coating with 20-25μm in thickness was formed on the outer surface. Pamela et al[37] applied this process to porous Ti6Al4V implants. A two-CaP-layer coating (an OCP layer inside and a high crystalline OCP layer outside) was successfully deposited on porous Ti6Al4V substrate as shown in Fig.3. However, the thickness of the coating was not the same throughout the implant. It varied between 20μm at the interior of the implant and 60μm at the implant periphery. Large OCP crystals were oriented perpendicularly to the surface of the metal.
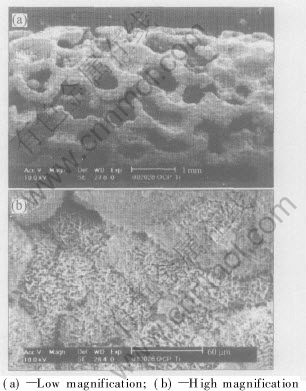
Fig.3 ESEM photographs of OCP-coated porous Ti6Al4V implant[37]
Histological examination of the in-vivo dog studies has shown that new bone was formed in the gap between the biomimetically Ca-P coated titanium implant and the bone after 4 weeks implantation. On the other hand, only an intervening fibrous layer or a small amount of bone was in direct contact with the untreated titanium implant[39].
Animal studies have shown that after 4 weeks implantation abundant bone in-growth into the centre of the biomimetic Ca-P coated porous polymer implant was observed in all sections[36]. The coating fragments were integrated into new bone tissue. Comparative studies on the biological response to the uncoated and coated Ti-6Al-4V porous implant have shown that in the total implant area OCP coated Ti6Al4V gave a significantly higher amount of bone compared with the uncoated Ti6Al4V after 6 weeks and 12 weeks implantations, as shown in Fig.4. However, in cortical area, no significant difference existed after 12 weeks implantation for bone contact. Measurements of the bone contact in inner and outer zone of the cortical area showed a significantly higher bone content in the outer zone than in the inner zone after 6 weeks of implantation for each kind of implant. After 12 weeks, no such difference could be found

Fig.4 Histomorphometrical results of percentage of bone contact(a) and percentage of bone in total implant area(b)[37]
any more.
In-vivo rabbit study[26] on the bone bonding at the bone/implant interface of alkali- and heat-treated and untreated tantalum implants indicated that the treated implants showed weak bonding to bone after 8 weeks, and exhibited significantly higher tensile failure loads compared with untreated tantalum implants after 16 weeks. In contrast, the untreated implants showed almost no bonding, even after 16 weeks. The bone-bonding shear strengths of the alkali- and heat-treated and untreated Ti implants measured by push-out test showed that after 4 weeks all types of the alkali- and heat-treated implants showed significantly higher bonding strength (2.4-4.5MPa) than their untreated counterparts (0.3-0.6MPa)[39], as shown in Fig.5. After 12 weeks the bonding strength of the treated implants showed no further increase, while that of the untreated implants had increased to 0.6-1.2MPa.

Fig.5 Results of push-out strength between bone and Ti alloy substrates after 4 weeks implantation(a) and 12 weeks implantation(b)[39]
All results showed that the biomimetic apatite coating could promote an early and strong bonding to bone without intervening fibrous tissue or promote the bone in-growth into the porous structure, displaying a very good osteoconductivity, especially at the early stage, which could be useful in establishing cementless stable fixation of orthopaedic implants. It is suggested that the biomimetic coating technique has the potential to become an accepted method of promoting and enhancing the bone-bonding abilities of metal orthopedic implants although further studies are needed to determine the optimal conditions for the biomimetic coating, and evaluate the biological and mechanical properties of the Ca-P coating.
5 CONCLUSIONS
As a surface coating technique, biomimetic has shown significant advantage in the preparation of calcium phosphate coating on metal and polymer substrate for biomedical application, including low cost and flexible process, wide range of apatite composition and thickness, and non-line-of-sight characteristic. Due to the physiological conditions, it is possible to coat heat-sensible materials such as polymers, and to cover complex shaped materials, especially the porous implants. Furthermore, this technique allows the covering of implants with new Ca-P phases that can not be produced at high temperatures. These new biomimetic coatings have different structures and dissolution behaviour depending on their crystal size and phase composition. In-vitro and in-vivo studies have shown that the biomimetic apatite coating could promote an early and strong bonding to bone without intervening fibrous tissue or promote the bone in-growth into the porous structure, showing a very good osteoconductivity, especially at the early stage, which will be useful in establishing cementless stable fixation of orthopaedic implants. However, the bonding strength between the apatite coating and the substrate is still low, and much research has to be done in order to apply this technology to the practice.
ACKNOWLEDGEMENT
One of authors (ZHANG Er-lin) would like to acknowledge the financial support (Program AM07-YC13) from Institute of Metal Research (IMR), Chinese Academy of Sciences (CAS), Shenyang, China.
REFERENCES
[1]Noort R V. Titanium: the implant material of today[J]. J Mater Sci, 1987, 22: 3801-3811.
[2]Niinomi M. Recent metallic materials for biomedical applications[J]. Metallurgical and Materials Transactions A, 2002, A33: 477-486.
[3]Kokubo T, Kim H M, Kawashita M. Novel bioactive materials with different mechanical properties[J]. Biomaterials, 2003, 24: 2167-2175.
[4]Kuroda D, Niinomi M, Morinaga M, et al. Design and mechanical properties of new β type titanium alloys for implant materials[J]. Mater Sci Eng, 1998, A243: 244-249.
[5]Song Y, Xu D S, Yang R, et al. Theoretical study of the effects of alloying elements on the strength and modulus of β-type bio-titanium alloys[J]. Mater Sci Eng, 1999, A260: 269-274.
[6]Tsui Y C, Doyle C, Clyne T W. Plasma sprayed hydroxyapatite coatings on titanium substrates. Part 2: optimisation of coating properties[J]. Biomaterials, 1998, 19: 2031-2043.
[7]Guipont V, Espanol M, Borit F, et al. High-pressure plasma spraying of hydroxyapatite powders[J]. Mater Sci and Eng, 2002, A325: 9-18.
[8]Ding S J, Su Y M, Ju C P, et al. Structure and immersion behavior of plasma-sprayed apatite-matrix coatings[J]. Biomaterials, 2001, 22: 833-845.
[9]Wei M, Ruys A J, Swain M V, et al. Interfacial bond strength of electrophoretically deposited hydroxyapatite coatings on metals[J]. J Mater Sci Mater Med, 1999, 10: 401-409.
[10]Zhitomirsky I, Gal-Or L, Electrophoretic deposition of hydroxyapatite[J]. J Mater Sci Mater Med, 1997, 8: 213-219.
[11]Wang J, Layrolle P, Stigter M, et al. Biomimetic and electrolytic calcium phosphate coatings on titanium alloy: physicochemical characteristics and cell attachment[J]. Biomaterials, 2004, 25: 583-592.
[12]Barrere F, van Blitterswijik C A, de Groot K, et al. Influence of ionic strength and carbonate on the Ca-P coating formation from SBFx5 solution[J]. Bio-materials, 2002, 23: 1921-1930.
[13]Liu Y, Layrolle P. De B J, et al. Biomimatic co-precipitation of calcium phosphate and bovine serum albumin on titanium alloy[J]. J Biomed Mater Res, 2001, 57(3): 327-335.
[14]Kumar M, Dasarathy H, Riley C. Electrodeposition of brushite coatings and its transformation to hydroxyapatite in simulated body fluid[J]. J Biomed Mater Res, 1999(45): 302-310.
[15]Helen A, Therese G, Vishnu K, et al. Novel electrosynthetic rout to calcium phosphate coatings[J]. J Mater Chem, 1998(8): 405-408.
[16]Ban S, Maruno S. Morphology and microstructure of electrochemically deposited calcium phosphate in a modified simulated body fluid[J]. Biomaterials, 1998(19): 1245-1253.
[17]Ban S, Maruno S, Arimoto N, et al. Effect of electrochemically deposited apatite coating on bonding of bone to the HA-G-Ti composite and titanium[J]. J Biomed Mater Res, 1997(36): 9-15.
[18]ZHANG E L, YANG K. Coating of calcium phosphate on biometallic materials by electrophoretic deposition: a review[J]. Non-ferrous Metals, in press.
[19]Li P, Ducheyne P. Quasi-biological apatite film induced by titanium in a simulated body fluid[J]. J Biomed Mater Res, 1998, 41: 341-348.
[20]Kim H M, Miyaji F, Kokubo T, et al. Apatite forming ability of alkali-treated Ti metal in body environment[J]. J Ceram Soc Jpn, 1997, 105: 111- 116.
[21]Kokubo T, Miyaji F, Kim H M, et al. Spontaneous apatite formation on chemically treated titanium metals[J]. J Am Ceram Soc, 1996, 79: 1127-1129.
[22]Kokubo T. Apatite formation on surfaces of ceramics, metals and polymers in body environment[J]. Acta Mater, 1998, 46: 2519-2527.
[23]Kim H M, Miyaji F, Kokubo T, et al. Preparation of bioactive Ti and its alloys via simple chemical surface treatment[J]. J Biomed Mater Res, 1996, 32: 409- 417.
[24]Wei M, Kim H M, Kokubo T, et al. Optimizing the bioactivity of alkaline-treated titanium alloy[J]. Materials Science and Engineering C, 2002, 20: 125-134.
[25]Kato H, Nakamura T, Nishiguchi S, et al. Bonding of alkali- and heat-treated tantalum implants to bone[J]. J Biomed Mater Res(Appl Biomater), 2000, 53: 28-35.
[26]Kokubo T, Kim H M, Kawashita M, et al. Bioactive metals: preparation and properties[J]. J Mater Sci Mater Med, 2004, 15: 99-107.
[27]Liang F, Zhou L, Wang K. Apatite formation on porous titanium by alkali and heat treatment[J]. Surface and Coating Tech, 2003, 165: 133-139.
[28]Jonasova L, Muller F A, Helebrant A, et al. Bio-mimetic apatite formation on chemically treated titanium[J]. Biomaterials, 2004, 25: 1187-1194.
[29]Jonasova L, Muller F A, Helebrant A, et al. Hydroxyapatite formation on alkali-treated titanium with different content of Na+ in the surface layer[J]. Biomaterials, 2002, 23: 3095-3101.
[30]Hamadaa K, Kon M, Hanawa T, et al. Hydrothermal modification of titanium surface in calcium solutions[J]. Biomaterials, 2002, 23: 2265-2272.
[31]Barrere F, Layroll P, van Blitterswijk C A, et al. Fast formation of biomimetic Ca-P coating on Ti6Al4V[J]. Mater Res Soc Symp Proc, 2000, 599: 135-140.
[32]Barrere F, Layroll P, van Blitterswijk C A, et al. Biomimetic calcium phosphate coatings on titanium: a crystal growth study of octacalcium phosphate[J]. J Mater Sci Mater Med, 2001, 12: 529-534.
[33]Barrere F, van Blitterswijk C A, de Groot K, et al. Nucleation of biomimetic Ca-P coatings on Ti6A14V from a SBFX5 solution: influence of magnesium[J]. Biomaterials, 2002, 23(10): 2211-20.
[34]Barrere F, Snel M E, van Blitterswijk C A, et al. Nano-scale study of the nucleation and growth of calcium phosphate coating on titanium implants[J]. Biomaterials, 2004, 25: 2901-2910.
[35]Liu Y, Junziker E B, Randall N X, et al. Proteins incorporated into biomimetically prepared calcium phosphate coatings modulate their mechanical strength and dissolution rate[J]. Biomaterials, 2003, 24: 65-70.
[36]Du C, Meijerc G J, van de Valk C, et al. Bone growth in biomimetic apatite coated porous Polyactive 1000 PEGT70PBT30 implants[J]. Biomaterials, 2002, 23: 4649-4656.
[37]Habibovic P, Li J P, van der Valk C M, et al. Biological performance of uncoated and octacalcium phosphate-coated Ti6Al4V[J]. Biomaterials, 2005, 26: 23-36.
[38]Barrere F, van der Valk C M, Dalmeijer R A J, et al. Osteogenecity of octacalcium phosphate coatings applied on porous metal implants[J]. J Biomed Mater Res, 2003, A66: 779-788.
[39]Nishiguchi S, Kato H, Fujita H, et al. Titanium metals form direct bonding to bone after alkali and heat treatments[J]. Biomaterials, 2001, 22: 2525-2533.
(Edited by YANG Bing)
Received date: 2004-12-23; Accepted date: 2005-08-16
Correspondence: ZHANG Er-lin, Professor, PhD; Tel/Fax: +86-24-23971605; E-mail: erlin.zhang@imr.ac.cn