J. Cent. South Univ. Technol. (2008) 15: 679-683
DOI: 10.1007/s11771-008-0126-4

Leaching kinetics of low grade zinc oxide ore in
NH3-NH4Cl-H2O system
WANG Rui-xiang(王瑞祥)1, 2, TANG Mo-tang(唐谟堂)1, YANG Sheng-hai(杨声海)1,
ZHAGN Wen-hai(张文海)1, TANG Chao-bo(唐朝波)1,
HE Jing(何 静)1, YANG Jian-guang(杨建广)1
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Materials and Chemical Engineering, Jiangxi University of Science and Technology,
Ganzhou 341000, China)
Abstract: The leaching kinetics of low grade zinc oxide ore in NH3-NH4Cl-H2O system was studied. The effects of ore particle size, reaction temperature and the sum concentration of ammonium ion and ammonia on the leaching efficiency of zinc were examined. The leaching kinetics of low-grade zinc oxide ore in NH3-NH4Cl-H2O system follows the kinetic law of shrinking-core model. The results show that diffusion through the inert particle pores is the leaching kinetics rate controlling step. The calculated apparent activation energy of the process is about 7.057 kJ/mol. The leaching efficiency of zinc is 92.1% under the conditions of ore particle size of 69 μm, holding at 80 ℃ for 60 min, sum ammonia concentration of 7.5 mol/L, the molar ratio of ammonium to ammonia being 2?1, and the ratio (g/mL) of solid to liquid being 1?10.
Key words: NH3-NH4Cl-H2O system; low-grade zinc oxide ore; leaching; kinetics
1 Introduction
The increasing demand for zinc has required intensive studies on new hydrometallurgical processes for extracting zinc from low-grade oxide ores. Leaching zinc oxide ore by H2SO4 or HCl and its kinetics have been studied by some researchers[1-4]. Leaching low-grade zinc oxide ore consumes a great deal of acid because of existence of large amounts of basic gangue such as CaO and MgO in it. At the same time, impurities such as Fe and Si are also leached, which causes adverse effect on the subsequent handling. So zinc oxide ore has been treated in an alkaline medium[5]. Ammonia metallurgical processes were conducted in the alkaline medium, and because of the advantages, people put more emphases on them in recent years. Leaching zinc sulphide concentrate by concentrate ammonium chloride has been used in industry[6]. YANG et al[7] researched the treatment of zinc oxide ore for producing zinc in the system of NH3-NH4Cl, and the thermodynamics of the system was also studied[8-9]. Leaching is the key step in the hydrometallurgical process, and the leaching kinetics is very important to economically extract zinc. There are few studies on leaching kinetics of zinc oxide ore by NH3-NH4Cl-H2O system. The kinetics of leaching zinc oxide ore with NH3·H2O has been studied by some researchers[10]. JU et al[11] studied the kinetics leaching smithsonite with NH4Cl solution. In the process NH3 needed to form zinc ammonia complex was supplied by the decomposition of NH4+. But the velocity of these reactions is slow. To study the kinetics of ammonia leaching zinc oxide ore, the leaching kinetics of low-grade zinc oxide ore in the NH3-NH4Cl-H2O system was investigated in this work.
2 Principle and kinetic model
2.1 Principle
Leaching process consists of five steps as follows.
1) Transport of the reagent (NH3) from the bulk solution to the particle surface.
2) Diffusion of the reagent (NH3) through the solid residual layer from the particle surface to the surface of unreacted core.
3) Reaction between the reagent (NH3) and the zinc oxide ore on the surface (reaction interface) of the unreacted core. The reactions can be described as follows:
ZnO+iNH3+H2O=[Zn(NH3)i]2++2OH- (1)
ZnCO3+iNH3=[Zn(NH3)i]2++CO32- (2)
ZnSO4+iNH3=[Zn(NH3)i]2++SO42- (3)
where i=1, 2, 3, 4.
4) Diffusion of the resultants through the solid residual layer from the reaction interface to the particle surface.
5) Transport of the resultants from the particle surface to the bulk solution.
In these steps, steps 2 and 4 are diffusion steps in the solid residual layer, and they may be the control steps.
2.2 Kinetic model
In the leaching process, solid particles almost remain unchanged in size during the heterogeneous reaction if the ore contains large amounts of gangues, which remain as a nonflaking residual so called solid layer. The thickness of insoluble solid residual layer progressively increases as the reaction proceeds, while the size of unreacted inner core decreases. It results in the reduction of the reactant surface and the increase in path length for the diffusion of ions[12-13]. The analysis results of the ore and leached residue by scanning electron microscope(SEM) show that the difference between their sizes is very little. This shows that the kinetics of the leaching process follows the shrinking core model. According to the model, the reaction firstly occurs on the outer surface of the solid particles and this surface shrinks towards the center of the sphere as the reaction proceeds, leaving behind a solid layer around the unreacted shrinking core[14-15]. According to this model, if the rate of the reaction is controlled by the step of diffusion through solid layer consisted of insoluble part of the ore around the unreacted core, the integral rate equation is as follows:
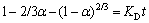 (4)
(4)
where α is the leaching efficiency of zinc; KD is the pore diffusion rate constant; t is the reaction time.
If the reaction rate is controlled by chemical reaction, then the integral rate expression becomes
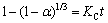 (5)
(5)
where KC is the surface chemical rate constant.
3 Experimental
3.1 Materials and reagents
The low-grade zinc oxide ore used in this study was taken from Yunnan Province of China. The ore was ground and sieved. Its chemical compositions are listed in Table 1. Table 2 lists the mineralogical composition of zinc of the ore. It can be seen from Table 1 that the ore contains 19.510% Zn and major gangue consists of SiO2, Fe, Al2O3, MgO and CaO. It can be seen from Table 2 that the major zinc bearing mineral is hydrozincite.
Table 1 Chemical compositions of ore (mass fraction, %)
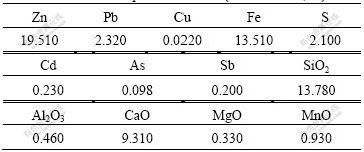
Table 2 Mineralogical composition of zinc in ore (mass fraction, %)

Ammonium chloride of the industry grade with content of 99.2% and ammonia water of the analytical-reagent grade with a content of 98% were produced by Guangdong Xilong Chemical Co, Ltd, China.
3.2 Experimental apparatus
Experimental apparatus is shown in Fig.1. The experiment was performed in a round-bottom three- necked flask of 1 000 mL. The reaction temperature was maintained constant with the temperature error of ±1 ℃ controlled by a constant temperature magnetic stirrer. To make the leaching agent homogeneously, the agitate rate was chosen to be 450 r/min.
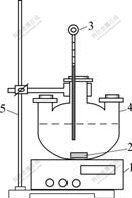
Fig.1 Equipment apparatus sketch: 1—Constant temperature magnetic stirrer; 2—Agitator; 3—Thermometer; 4—Three- necked flask; 5—Iron stand
3.3 Experimental methods
A solution of ammonium chloride and ammonia water 100 mL were put into the reactor. When the solution reached the required temperature, 10 g ore powders with required size was added. At selected time intervals, a solution sample of about 1 mL was taken out using a syringe filter. The zinc ion concentration of the sample was measured by volumetric titration analysis with EDTA.
4 Results and discussion
4.1 Effect of particle size on leaching efficiency of zinc
The effect of particle size on the leaching efficiency of zinc was studied under the conditions that sum concentration of initial ammonium ion and ammonia and leaching temperature were kept constant of 7.5 mol/L and 40 ℃, respectively, the leaching time was 60 min, the molar ratio of ammonium to ammonia was 2?1 and the ratio (g/mL) of solid to liquid was 1?10. The results are given in Fig.2. These results show that about 88.9% of zinc in the ore with particle size of 69 ?m is extracted. When the initial particle size of the ore increases to 98 ?m, the leaching efficiency of zinc decreases to 76.72%. These indicate that the leaching velocity of zinc increases as the particle size of the ore decreases. The smaller the particle size of the ore sample, the faster the leaching velocity of zinc, and the higher the leaching efficiency. The reason is that total quantum of ore sample is constant. The number of particles increases as the particle size of the ore decreases and then the reaction-surface area increases. The leaching velocity is in direct proportion to the reaction-surface area. So the leaching velocity and the leaching efficiency of zinc increase as the particle size of the ore sample decreases.

Fig.2 Effects of particle size on leaching efficiency of zinc
Substituting the experimental results into Eqns.(4) and (5), the relationship between 1-2/3α-(1-α)2/3 and leaching time at different particle sizes is shown in Fig.3. There is good linear relationship between 1-2/3α- (1-α)2/3 and leaching time. It is determined that leaching process is controlled by the step of diffusion through the solid layer around the shrinking unreacted core. So the leaching kinetics can be described by the diffusion model.
4.2 Effect of reactant concentration on leaching efficiency of zinc
The effect of the sum concentration of ammonium ion and ammonia on the leaching efficiency of zinc was studied under the conditions that fine ore particle size was 69 ?m, the leaching temperature was 40 ℃, the leaching time was 60 min, the molar ratio of ammonium to ammonia was 2?1, and the ratio (g/mL) of solid to liquid was 1?10. The results are shown in Fig.4. It is obvious that the sum concentration of ammonium ion and ammonia has a significant effect on the dissolution of zinc. Leaching velocity and leaching efficiency of zinc both increase as the sum concentration of ammonium ion and ammonia increase. About 88.9% and 84.31% of zinc from the ore are extracted using 7.5 mol/L and 4.5 mol/L sum concentrations of ammonium ion and ammonia, respectively. When the sum concentration decreases to 1.5 mol/L, leaching efficiency of zinc decreases and only 59.4% of zinc is extracted from the sample.
Substituting the results into Eqn.(4), the relationship between 1-2/3α-(1-α)2/3 and leaching time at different concentrations of leaching agent is shown in Fig.5.

Fig.3 1-2/3α-(1-α)2/3 vs time at different particle sizes

Fig.4 Effect of sum ammonia concentration on leaching efficiency of zinc

Fig.5 1-2/3α-(1-α)2/3 vs time at different concentrations of leaching agent
As shown in Fig.5, there is also a linear relationship between leaching time and the diffusion model fraction. This proves again that the leaching kinetics can be described by the diffusion model.
4.3 Effect of reaction temperature on leaching efficiency of zinc
The effect of reaction temperature on the leaching efficiency of zinc was studied under the conditions that sample particle size was 69 ?m and sum ammonia concentrations was 7.5 mol/L, the leaching time was 60 min, the molar ratio of ammonium to ammonia was 2?1 and the ratio (g/mL) of solid to liquid was 1?10. The results are shown in Fig.6. Fig.6 shows that the reaction temperature has an unnoticeable effect on the dissolution of zinc. When the reaction temperature increases form 40 to 80 ℃, the leaching efficiency of zinc only increases 3.1% (from 88.9% to 92.1%). In the view of that volatilization rate of ammonia increases as the temperature increases, lower operating temperature is suitable adopted to reduce reagent consumption.
Based on the experimental data in Fig.6, the relationship between 1-2/3α-(1-α)2/3 and time is given

Fig.6 Effect of temperature on leaching efficiency of zinc
in Fig.7. Figs.6 and 7 show that about 90.6% of zinc in the ore sample is extracted at 80 ℃ for 40 min. When leaching time increases from 40 to 60 min, the leaching efficiency increases slowly to 92.1%. When the reaction time increases from 50 to 60 min, the leaching efficiency only increases form 90.5% to 91.2% at 60 ℃. Because the leaching efficiency has reached the maximum, the last two points at 80℃ and the last point at 60 ℃ are omitted (in Fig.7). The plot in Fig.7 is also linear, which indicates that the rate of the reaction is controlled by diffusion through the solid layer around the shrinking unreacted core.
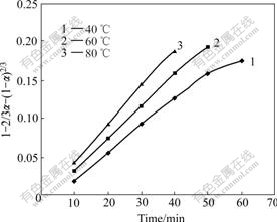
Fig.7 1-2/3α-(1-α)2/3 vs time at different temperatures
4.4 Kinetic model analysis
4.4.1 Diffusion control model
Based on Figs.3, 5, 7 and the above analyses, it can be concluded that under the experimental conditions, the leaching kinetics can be described by diffusion model, and controlled by the step of diffusion through the solid residual layer around the shrinking unreacted core.
4.4.2 Calculation of activation energy
To calculate the activation energy, the Arrhenius plot of this leaching process is plotted using the values of ln KD against 1/T in Fig.8. The apparent activation energy

Fig.8 Arrhenius plot for activation energy
of the overall reaction is calculated to be about 7.057 kJ/mol. It proves earlier description of the rate control step.
5 Conclusions
1) Zinc in low-grade zinc oxide ore can be leached in the system of NH3-NH4Cl-H2O. Leaching efficiency of zinc is 92.1% under the conditions that particle size is 69 ?m, sum ammonia concentration is 7.5 mol/L, the molar ratio of ammonium to ammonia is 2?1, and the ratio (g/mL) of solid to liquid is 1?10 at 80 ℃ for 60 min.
2) Leaching process is controlled by the diffusion step through the solid residual layer around the shrinking unreacted core. Leaching velocity can be described by the shrinking core model. The calculated apparent activation energy is about 7.057 kJ/mol.
3) Increasing the concentration of leaching agent properly, raising reaction temperature and decreasing the particle size of the ore can increase the leaching efficiency of zinc.
References
[1] QIN Wen-qing, LAN Zhuo-yue, LI Wei-zhong. Recovery of zinc from low-grade zinc oxide ores by solvent extraction [J]. J Cent South Univ Technol, 2003, 10(2): 98-102.
[2] HE Jing, TANG Mo-tang, LU Jun-le, LIU Zhong-qing, YANG Sheng-hai, YAO Wei-yi. Concentrating Ge in zinc hydrometallurgical process with hot acid leaching-halotrichite method [J]. J Cent South Univ Technol, 2003, 10(4): 307-312.
[3] ABDEL-AAL E A. Kinetics of sulfuric acid leaching of low-grade zinc silicate ore [J]. Hydrometallurgy, 2000, 39(2): 247-254.
[4] IGINIO C, SERGIO M, UMBERTO M. Kinetic models for the leaching with sulphuric acid of zinc-containing slags [J]. Hydrometallurgy, 1983, 10(1): 61-67.
[5] LIU San-jun, OU Le-ming, FENG Qi-min, ZHANG Guo-fan, L? Yi-ping. Alkaline leaching Zn from zinc oxide ore [J]. Hydrornetallurgy of China, 2005, 24(1): 23-25. (in Chinese)
[6] LIMPO J L, FIGUEIREDO J M, AMER S, LUIS A. The CENIM-LNETI process: A new process for the hydrometallurgical treatment of complex sulphides in ammonium chloride solutions [J]. Hydrometallurgy, 1992, 28(2): 149-161.
[7] YANG Sheng-hai, TANG Mo-tang, CHEN Yi-feng, TANG Chao-bo, HE Jing. Anodic reaction kinetics of electro winning zinc in system of Zn(II)-NH3-NH4Cl-H2O [J]. Trans Nonferrous Met Soc China, 2004, 14(3): 626-630.
[8] YANG Sheng-hai, TANG Mo-tang. Thermodynamics of Zn(Ⅱ)-NH3-NH4Cl-H2O system [J]. Trans Nonferrous Met Soc China, 2000, 10(6): 830-833.
[9] LIMPO J L, LUIS A. Solubility of zinc chloride in ammoniacal ammonium chloride solutions [J]. Hydrometallurgy, 1993, 32(2): 247-260.
[10] ZHU Yun, HU Han, SU Yun-sheng, YANG Bao-min. Kinetics of leaching poorly-floated zinc-oxide-ore with ammonia [J]. The Chinese Journal of Process Engineering, 2002, 2(1): 81-85. (in Chinese)
[11] JU Shao-hua, TANG Mo-tang, YANG Sheng-hai, LI Ying-nian. Dissolution kinetics of smithsonite ore in ammonium chloride solution [J]. Hydrometallurgy, 2005, 80(1): 67-74.
[12] XUIN G H, YU D Y, SU Y F. Leaching of Scheelite by hydrochloric acid in the presence of phosphate [J]. Hydrometallurgy, 1986, 16(1): 27-40.
[13] EKMEKYAPAR A, OYA R. Dissolution kinetics of an oxidized copper ore in ammonium chloride solution [J]. Chemical and Biochemical Engineering Quarterly, 2003, 17(4): 261-266.
[14] MULAK W, MIAZGA B, SZYMLZYCHA A. Kinetics of nickel leaching from spent catalyst in sulphuric acid solution [J]. Internation Journal of Mineral Processing, 2005, 77(4): 231-235.
[15] KUNKUL A, MUHTAR K M, YAPICI M, DEMIRBA A. Leaching kinetics of malachite in ammonia solutions [J]. Mineral Processing, 1994, 41(3): 167-182.
(Edited by ZHAO Jun)
Foundation item: Project(2007CB613604) supported by the Major State Basic Research Development Program of China; Project(50674104) supported by the National Natural Science Foundation of China; Project(GJJ08279) supported by the Department of Education of Jiangxi Province
Received date: 2007-10-13; Accepted date: 2007-12-25
Corresponding author: WANG Rui-xiang, PhD; Tel: +86-731-8830470; E-mail: wrx9022@163.com