采用硫化砷渣制备三氧化二砷工艺
郑雅杰,刘万宇,白 猛,张传福
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘 要:硫化砷渣经氢氧化钠溶液浸出、空气氧化脱硫和SO2还原制备得到As2O3。研究结果表明:当NaOH与As2S3物质的量比为7.2?1,固体质量与液体体积之比为1?6,反应温度为90 ℃,反应时间为2 h,转速为300 r/min时,用氢氧化钠溶液浸取硫化砷渣,其砷的浸取率达到95.90%;过滤后在碱浸液中通空气脱除碱浸液中Na3AsS3中的硫;当反应时间为10 h,反应温度为30 ℃,空气流量为120 L/h,对苯二酚和高锰酸钾质量浓度分别为1.5 g/L和0.5 g/L,木质素磺酸钠质量浓度为0.13 g/L时,脱硫率可达到96.00%;当pH值为0,反应时间为1 h,反应温度为30 ℃,砷质量浓度为60.00 g/L时通入SO2还原溶液中AsO43-,产物中As2O3含量和砷回收率分别达到92.14%和95.21%;稀硫酸洗涤后,As2O3纯度达95.14%。
关键词:硫化砷渣;碱浸;氧化;还原;三氧化二砷
中图分类号:TF09 文献标识码:A 文章编号:1672-7207(2008)06-1157-07
Preparation of arsenic trioxide from arsenic sulfide slag
ZHENG Ya-jie, LIU Wan-yu, BAI Meng, ZHANG Chuan-fu
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Arsenic trioxide was prepared by leaching arsenic sulfide slag in NaOH solution, oxidating with air and reducing with sulfur dioxide. The results show that arsenic sulfide slag is leached by sodium hydroxide solution and the leaching rate of arsenic is 95.90% when the molar ratio of NaOH/As2S3 is 7.2?1, the ratio of mass of solid to volume of liquid is 1?6, reaction temperature is 90 ℃, reaction time is 2 h, and agitation speed is 300 r/min. After filtration, the sulphur of Na3AsS3 is removed by blowing air into the alkali leaching solution. The removal rate of sulphur is 96.00%, when reaction time is 10 h, reaction temperature is 30 ℃, ventilation flux is 120 L/h, hydroquinone is 1.5 g/L, potassium permanganate is 0.5 g/L and lignin is 0.13 g/L. When pH value is 0, reaction time is 1 h, reaction temperature is 30 ℃, the concentration of As is 60 g/L, arsenic trioxide content of the product and recycling rate of As are 92.14% and 95.21%, respectively, after AsO43- in the solution is reduced by SO2. After washing in dilute sulphuric acid, the purity of As2O3 is 95.14%.
Key words: arsenic sulfide slag; alkali leaching; oxidation; reduction; arsenic trioxide
As2O3是一种剧毒物质,0.1 g可致人死亡,人长期接触还可致癌[1]。As2O3可用作玻璃工业的澄清脱色剂[2],生产高纯砷,在医药、制革、印染等行业中也得到一定应用[3-4]。三氧化二砷的制备方法有火法和湿法。火法包括焙烧法、熔炼法和蒸馏法[5-6],对操作人员健康危害大,能耗高,现已基本被淘汰。湿法主要有硫酸铜置换法、加压氧化还原法[7]和硫酸铁法[8-9],硫酸铜置换法的铜粉消耗量大,加压氧化还原法对设
备要求较高,硫酸铁法工艺复杂。碱浸液氧化还原法是碱性浸出硫化砷渣,使铜、铋与砷分离,铜和铋得到富集,在碱性浸出液中通空气得到单质硫,再通SO2还原得到三氧化二砷。该方法生产安全、工艺简单、无污染,能耗较低。本文作者研究氢氧化钠溶液浸出硫化砷渣、空气氧化脱硫和SO2还原工艺制备As2O3。
1 实 验
1.1 实验步骤
实验取某铜冶炼厂硫化钠沉淀含砷废水得到的硫化砷渣,其成分如表1所示。
表1 硫化砷渣化学成分
Table 1 Components of arsenic sulfide slag w/%

使用氢氧化钠溶液浸取硫化砷渣,经过过滤、洗涤得到碱浸渣和碱浸液。在碱浸液中通入空气氧化脱硫后过滤,在滤液中通入SO2气体还原,经加热蒸发、浓缩至还原液中砷为60 g/L后过滤、洗涤、烘干得到三氧化二砷粉末。其工艺流程如图1所示。
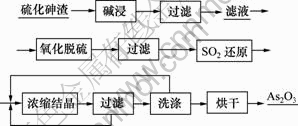
图1 硫化砷渣制备三氧化二砷的工艺流程
Fig.1 Process flow of preparation of arsenic trioxide from arsenic sulfide slag
1.2 分析与检测
实验采用溴酸钾滴定法[10]测定溶液中砷和固体产物中的砷含量。
根据化学反应物质的量关系,按如下公式计算脱硫率(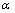 ):
):


2 结果与讨论
2.1 硫化砷渣的碱性浸取
实验取1 000 g 硫化砷渣,采用氢氧化钠溶液浸取。当NaOH与As2S3物质的量比为7.2?1,固体质量与液体体积比为1?6,反应温度为90 ℃,反应时间为2 h,转速为300 r/min时,砷浸出率为95.90%。浸取所得碱浸渣和碱浸液成分如表2和表3所示,其中,碱浸液pH值为7.8。
表2 碱浸渣化学成分
Table 2 Components of alkaline leached slag w/%

表3 硫化砷渣的碱浸液主要元素含量
Table 3 Components of alkaline leaching solution of arsenic sulfide slag ρ/(g?L-1)

由表2可知,硫化砷渣经过碱性浸取,Cu和Bi等与As得到有效分离。Cu和Bi等得到富集并可有效回收。由表3可知,As在碱浸液中质量浓度达到20 g/L,可提取回收砷。硫化砷渣在NaOH溶液中发生如下反应:
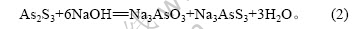
而硫化砷渣中CuS、Bi2S3不与NaOH反应。因此,Cu、Bi得到分离和富集。
2.2 碱浸液氧化脱硫
2.2.1 反应时间对脱硫率的影响
向400 mL碱浸液中以80 L/h的流量通空气,当对苯二酚质量浓度为1.5 g/L,反应温度为30 ℃时,反应时间对脱硫率的影响如图2所示。
由图2可知,脱硫率随氧化时间增加而增加,10 h时达到84.09%;随着氧化时间继续增加,脱硫率变化不大。在碱浸液中通入空气,发生如下反应[11]:
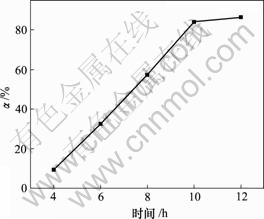
图2 反应时间对脱硫率的影响
Fig.2 Influence of reaction time on removing rate of sulphur
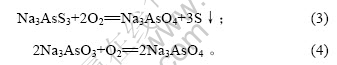
反应(3)为气液反应,30 ℃时氧气在溶液中的溶解度仅为水中空气溶解度的33.60%[12],扩散速率小[13],氧化速率慢。10 h后,溶液中生成的硫易结成膜,阻碍氧化,因此,反应时间对脱硫率影响不大。
2.2.2 反应温度对脱硫率的影响
当空气流量为80 L/h,对苯二酚质量浓度为1.5 g/L,反应时间10 h时,反应温度对脱硫率的影响如图3所示。
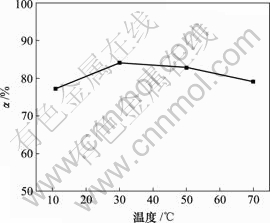
图3 反应温度对脱硫率的影响
Fig.3 Influence of reaction temperature on removing rate of sulphur
由图3可知,脱硫率随反应温度的升高先增加后降低。30 ℃时最大,为84.09%。因此,氧化脱硫的适宜温度为30 ℃。当反应温度高于50 ℃时,脱硫率降低,这是因为反应温度升高时,发生下列副反应[14]:

2.2.3 空气流量对脱硫率的影响
当对苯二酚质量浓度为1.5 g/L,反应时间10 h,反应温度为30 ℃时,空气流量对脱硫率的影响如图4所示。
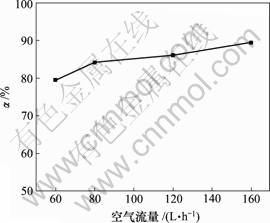
图4 空气流量对脱硫率的影响
Fig.4 Influence of air flux on removing rate of sulphur
由图4可知,脱硫率随空气流量的增大而增加,当空气流量为160 L/h时,脱硫率达89.33%,但增幅不明显。因此,空气流量为120 L/h比较合适,其脱硫率为85.96%。
2.2.4 对苯二酚用量对脱硫率的影响
当空气流量为120 L/h,反应时间10 h,反应温度为30 ℃时,对苯二酚用量对脱硫率的影响如图5所示。
图5 对苯二酚用量对脱硫率的影响
Fig.5 Influence of hydroquinone on removing rate of sulphur
由图5可知,脱硫率随对苯二酚质量浓度的增加而增加,对苯二酚质量浓度达1.5 g/L时,继续添加对苯二酚,脱硫率几乎不变。对苯二酚的载氧作用与质量浓度有一定关系[15],使用量较少时,载氧作用明显;过量的对苯二酚在一定条件下被空气氧化为醌,产生少量双氧水[14],可将少量S2-氧化为SO42-,降低脱 硫率。
为进一步提高脱硫率,使用1.5 g/L对苯二酚和0.5 g/L高锰酸钾的混合催化剂[15],脱硫率增加明显,可达95%,高锰酸钾起到一定的氧化作用。
2.2.5 表面活性剂实验
当空气流量为120 L/h,反应时间10 h,反应温度为30 ℃,对苯二酚质量浓度为1.5 g/L,高锰酸钾用量为0.5 g/L时,表面活性剂对脱硫率的影响如图6所示。
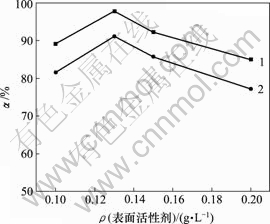
1—木质素磺酸钠;2—十二烷基磺酸钠
图6 不同表面活性剂及浓度对脱硫率的影响
Fig.6 Influence of surfactants and their concentration on removing rate of sulphur
由图6可知,表面活性剂浓度增加,脱硫率先增后减,0.13 g/L时,脱硫率最大,十二烷基磺酸钠为91.07%,木质素磺酸钠为97.67%,木质素磺酸钠的亲硫效果更好。表面活性剂亲油基会富集硫,破坏硫膜,增加与氧的接触,提高脱硫率。当表面活性剂过量时,在溶液中聚团[13, 16-18],降低反应速率。
上述实验表明,碱浸液氧化脱硫的适宜条件是反应时间为10 h,反应温度为30 ℃,空气流量为120 L/h,催化剂为1.5 g/L的对苯二酚和0.5 g/L的高锰酸钾,表面活性剂木质素磺酸钠为 0.13 g/L。
2.3 脱硫后溶液还原
在脱硫后溶液中通入SO2,主要反应为:
AsO43-+SO2+H+=SO42-+HAsO2 (8)
HAsO2溶解度较小,易脱水析出As2O3。
2.3.1 pH值对产物中As2O3含量和砷回收率的影响
在200 mL脱硫后溶液中通入SO2,当反应时间为40 min,反应温度为30 ℃,反应溶液中砷浓度为20.25 g/L时,pH值对产物中As2O3含量和砷回收率的影响如图7所示。
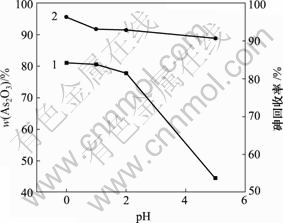
1—As2O3含量;2—砷回收率
图7 pH值对产物中As2O3含量和砷回收率的影响
Fig.7 Influence of pH on As2O3 content of product and recycling rate of As
由图7可知,产物中As2O3含量和砷回收率随着pH值升高而降低。pH值为0时产物中As2O3含量和砷回收率最大,分别为81.02%和96.35%。HAsO2的溶解度随pH值的减小而减小[19-20],pH值降低有利于产生的HAsO2析出。因此,pH值越低,产物中As2O3含量和砷回收率越高。
2.3.2 反应时间对产物中As2O3含量和砷回收率的 影响
当pH值为0,反应温度为30 ℃,反应物砷质量浓度为20.25 g/L时,反应时间对产物中As2O3含量和砷回收率的影响如图8所示。
由图8可知,随着反应时间的延长,产物中As2O3含量和砷回收率先增加后减少。当反应时间为1 h时,产物中As2O3含量和砷回收率达到最大,分别为89.67%和96.62%。溶液中随着反应时间的延长,杂质析出越多,导致产物中As2O3含量降低,砷回收率下降。
2.3.3 反应温度对产物中As2O3含量和砷回收率的 影响
当pH值为0,反应时间为1 h,砷质量浓度为20.25 g/L时,反应温度对产物中As2O3含量和砷回收率的影响如图9所示。

1—As2O3含量;2—砷回收率
图8 反应时间对产物中As2O3含量和砷回收率的影响
Fig.8 Influence of reaction time on As2O3 content of product and recycling rate of As
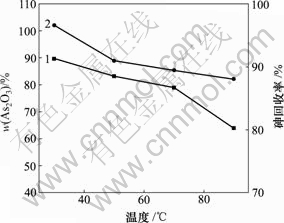
1—As2O3含量;2—砷回收率
图9 反应温度对产物中As2O3含量和砷回收率的影响
Fig.9 Influence of reaction temperature on As2O3 content of product and recycling rate of As
由图9可知,30 ℃时产物中As2O3含量和砷回收率最高,分别为89.67%和96.62%。随着反应温度的升高,产物中As2O3含量和砷回收率逐渐降低。反应(8)是放热反应[21],反应温度的升高不利于反应产物的生成。
2.3.4 砷浓度对产物中As2O3含量和砷回收率的影响
当 pH值为0,反应时间为1 h,反应温度为30 ℃时,砷浓度对产物中As2O3含量和砷回收率的影响如图10所示。
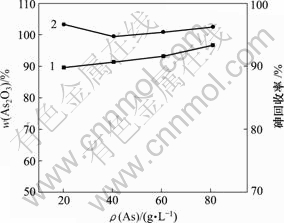
1—As2O3含量;2—砷回收率
图10 砷浓度对产物中As2O3含量和砷回收率的影响
Fig.10 Influence of As concentration on As2O3 content of product and recycling rate of As
由图10可知,随着反应物砷浓度的增加,产物中As2O3含量和砷回收率都逐渐升高,当砷质量浓度为81.00 g/L时,产物中As2O3含量和砷回收率较高,分别为96.64%和96.24%,此时,蒸发的能耗较高。HAsO2溶解度低[22];当砷质量浓度为60.00 g/L时,还原过程中会自动析出白色沉淀,纯度较高,能耗较低。
综上所述,氧化后液通SO2还原的适当条件是 pH值为0,反应时间为1 h, 反应温度为30 ℃,砷质量浓度为60.00 g/L。
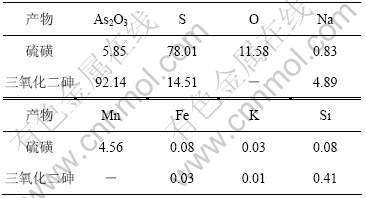
根据上述氧化脱硫以及还原的适宜条件进行放大实验。实验取碱浸液1 L进行氧化脱硫,再经浓缩、还原、过滤、洗涤、烘干制备得27.42 g As2O3。氧化脱硫得到的硫磺和还原产物三氧化二砷成分如表4所示,其X射线衍射实验结果如图11所示。
由图11可知,氧化脱硫产物为单质硫,还原产物为As2O3。根据表4可知,产物中As2O3含量为92.14%,砷回收率为95.21%。经稀硫酸洗涤后,As2O3纯度达95.14%。
表4 硫磺和三氧化二砷成分
Table 4 Components of sulphur and arsenic trioxide w/%

1—还原产物; 2—氧化产物
图11 氧化产物和还原产物的X射线衍射图
Fig.11 XRD patterns of oxidized product and reduced product
3 结 论
a. 在NaOH与As2S3物质的量比为7.2?1,固体质量与液体体积之比为1?6,反应温度为90 ℃,反应时间为2 h,转速为300 r/min的条件下,氢氧化钠溶液浸取硫化砷渣,砷浸取率达到95.90%,碱浸渣中Cu和Bi的质量分数分别为50.00%和10.63%。
b. 在反应时间为10 h,反应温度为30 ℃,空气流量为120 L/h,对苯二酚和高锰酸钾浓度分别为1.5 g/L和0.5 g/L,木质素磺酸钠质量浓度为0.13 g/L的条件下,脱硫率可达到96.00%。
c. 在pH值为0,反应时间为1 h,反应温度为30 ℃,溶液中砷浓度为60 g/L的适宜条件下,氧化后液通SO2,还原产物经稀硫酸洗涤,其中As2O3含量和砷回收率分别可达95.14%和95.21%。
d. X射线衍射证实氧化脱硫产物为硫磺,SO2还原产物为As2O3。
参考文献:
[1] Monhemius A J, Swash P M. Removing and stabilizing arsenic from copper refining circuits by hydrothermal processing[J]. Journal of Hazardous Materials, 1999, 51(9): 30-33.
[2] 刘小波, 章 礼, 杨名江. 含砷烟尘做玻璃澄清剂的研究[J]. 环境科学研究, 1995, 8(3): 46-47.
LIU Xiao-bo, ZHANG Li, YANG Ming-jiang. Use of arsenic smoke as the fining agent of glass[J]. Research of Environmental Sciences, 1995, 8(3): 46-47.
[3] 廖祥文. 含砷工业废水处理技术现状及展望[J]. 矿产综合利用, 2006, 8(4): 27-30.
LIAO Xiang-wen. Present situation and prospects of technology for treating As-containing industrial wastewater[J]. Multipurpose Utilization of Mineral Resources, 2006, 8(4): 27-30.
[4] 杨天足, 刘伟锋, 赖琼林, 等. 空气氧化法生产焦锑酸钠的氧化后液中砷和锑的脱除[J]. 中南大学学报: 自然科学版, 2005, 36(4): 576-581.
YANG Tian-zu, LIU Wei-feng, LAI Qiong-lin, et al. Removal of arsenic and antimony from oxidated solution of sodium thioantimonite production by air oxidation[J]. Journal of Central South University: Science and Technology, 2005, 36(4): 576-581.
[5] 吴俊升, 陆跃华, 周杨霁, 等. 高砷铅阳极泥水蒸焙烧脱砷实验研究[J]. 贵金属, 2003, 24(4): 26-31.
WU Jun-sheng, LU Yue-hua, ZHOU Yang-ji, et al. Experimental study on arsenic removal from arsenic-rich lead anode slime by volatilization roasting in steam condition[J]. Precious Metals, 2003, 24(4): 26-31.
[6] 仇勇海, 卢炳强, 陈白珍, 等. 无污染砷碱渣处理技术工业试验[J]. 中南大学学报: 自然科学版, 2005, 36(2): 234-237.
QIU Yong-hai, LU Bing-qiang, CHEN Bai-zhen, et al. Commercial scale test of anti-pollution control technique for slag of arsenic and soda[J]. Journal of Central South University: Science and Technology, 2005, 36(2): 234-237.
[7] Fermandez M A, Segarra M, Espiell F. Selective leaching of arsenic and antimony contained in the anode slimes from copper refining[J]. Hydrometallurgy, 1996, 41(2/3): 255-267.
[8] 覃用宁, 黎光旺, 何 辉. 含砷烟尘湿法提取白砷新工艺[J]. 有色冶炼, 2003, 32(3): 37-40.
QIN Yong-ning, LI Guang-wang, HE Hui. New wet process for extracting white arsenic from arsenic-bearing flue dust[J]. Non-ferrous Smelting, 2003, 32(3): 37-40.
[9] 董四禄. 湿法处理硫化砷渣研究[J]. 硫酸工业, 1994(5): 3-8.
DONG Si-lu. Research on treatment of arsenic sulphide cinder by a wet process[J]. Sulphuric Acid Industry, 1994(5): 3-8.
[10] 王维利, 车广华, 张英杰, 等. 氧化还原滴定法测定污染水中砷和锑[J]. 中国卫生检验杂志, 2001, 11(5): 580.
WANG Wei-li, CHE Guang-hua, ZHANG Ying-jie, et al. Determination of arsenic and antimony in the polluted water by oxidation-reduction titration[J]. Chinese Journal of Health Laboratory Technology, 2001, 11(5): 580.
[11] 金哲男, 蒋开喜, 魏绪钧, 等. 高温As-S-H2O系电位-pH图[J]. 矿冶, 1999, 12, 8(4): 45-50.
JIN Zhe-nan, JIANG Kai-xi, WEI Xu-jun, et al. Potential-pH diagrams of As-S-H2O system at high temperature[J]. Mining & Metallurgy, 1999, 12, 8(4): 45-50.
[12] 顾庆超, 楼书聪, 戴庆平, 等. 化学用表[M]. 江苏: 江苏科学技术出版社, 1979.
GU Qing-chao, LOU Shu-cong, DAI Qing-ping, et al. Chemical manual[M]. Jiangsu: Jiangsu Science Technology Press, 1979.
[13] 郭瑾龙, 程 文, 周孝德, 等. 小气泡扩散曝气氧传质速率研究[J]. 西安理工大学学报, 1999, 15(4): 86-90.
GUO Jin-long, CHENG Wen, ZHOU Xiao-de, et al. Research on the oxygen transfer rate of fine bubble diffused aeration in clean water[J]. Journal of Xi’an University of Technology, 1999, 15(4): 86-90.
[14] 寇建军, 朱昌洛. 硫化砷矿合理利用的湿法氧化新工艺[J]. 矿产综合利用, 2001, 6(3): 26-29.
KOU Jian-jun, ZHU Chang-luo. A new technology of aqueous oxidation for rational utililzation of arsenic sulfide ore[J]. Multipurpose Utilization of Mineral Resources, 2001, 6(3): 26-29.
[15] 施南赓, 贺小华. 几种工艺体系中离心传质机的传质性能[J]. 南京化工大学学报, 1999, 21(1): 39-43.
SHI Nan-geng, HE Xiao-hua. Mass transfer property of centrifugal mass transfer machine in some processes[J]. Journal of Nanjing University of Chemical Technology, 1999, 21(1): 39-43.
[16] 毕宝宽. 湿式氧化法脱硫过程副反应及腐蚀问题[J]. 小氮肥, 2006(11): 19-21.
BI Bao-kuan. The side reactions and corroding problem in the process of sulphur removing by hydro-oxidation[J]. Small Nitrogenous Fertilizer Plant, 2006(11): 19-21.
[17] 孙天友, 王吉坤, 杨大锦, 等. 硫化锌精矿加压氧化酸浸动力学研究[J]. 有色金属(冶炼部分), 2006(2): 21-23.
SUN Tian-you, WANG Ji-kun, YANG Da-jin, et al. Study on oxidative leading kinetics of sphalerite in acid solution under pressure[J]. Nonferrous Metals(Extractive Metallurgy), 2006(2): 21-23.
[18] 赵国玺, 朱步瑶. 表面活性剂作用原理[M]. 北京: 中国轻工业出版社, 2003.
ZHAO Bao-xi, ZHU Bu-yao. Function principle of surfactant[M]. Beijing: China Light Industry Press, 2003.
[19] Rusen M J. Surfactants and interfacial phenomena[M]. 2nd ed. New York: Wiley, 1989.
[20] Chukhlantsev V G. Solubility products of arsenates[J]. Journal of Inorganic of Chemistry (USSR), 1956(1): 1975-1982.
[21] 孙世连, 赵 文, 金思毅. 可逆放热反应过程流程结构的确定[J]. 化学工程, 2006, 34(1): 24-27.
SUN Shi-lian, ZHAO Wen, JIN Si-yi. Optimal design of reactor networks in reversible exothermic reaction system[J]. Chemical Engineering, 2006, 34(1): 24-27.
[22] 刘昌勇. 贵溪冶炼厂亚砷酸生产工艺[J]. 有色冶炼, 1982(2): 8-10.
LIU Chang-yong. Arsenous acid production in Guixi smelter[J]. Non-Ferrous Smelting, 1982(2): 8-10.
收稿日期:2008-01-10;修回日期:2008-03-17
基金项目:广东省创新基金资助项目(200501045)
通信作者:郑雅杰(1959-),男,湖南常德人,教授,从事湿法冶金、水污染控制、资源综合利用;电话:0731-8836285;E-mail: zzyyjj01@yahoo.com.cn