Versatic 10萃取体系和由Versatic 10 和Cyanex 301组成的复配体系萃取分离硫酸盐溶液中镍钴和钙镁
来源期刊:中国有色金属学报(英文版)2016年第3期
论文作者:管青军 孙伟 周桂英 刘佳鹏 殷志刚
文章页码:865 - 873
关键词:溶剂萃取;Verrsatic 10;Cyanex 301;镍;钴;镁;钙
Key words:solvent extraction; Versatic 10; Cyanex 301; nickel; cobalt; magnesium; calcium
摘 要:考察萃取剂Versatic 10 以及它和Cyanex 301组成的复配萃取体系从含有镁钙离子的硫酸盐溶液中萃取分离镍钴离子的萃取和反萃特征。Versatic 10萃取分离镍钴和钙镁的最佳条件为初始pH值为2.5、油水体积比O/A为1/3、皂化率为60%。Versatic 10的负载有机相用2 mol/L的硫酸溶液以O/A为1/1进行反萃。复配萃取体系中Versatic 10和Cyanex 301的最佳体积比为7/3,复配体系能有效降低镍钴和钙镁萃取分离的平衡pH值至3.50,当O/A为1/2时,2 mol/L的硫酸溶液可以对负载有机相进行有效反萃。Versatic 10与金属离子进行萃取的反应机理为离子交换,而Cyanex 301与金属离子之间不仅有离子交换还有强烈的配位效应,这种配位效应在复配体系与金属离子萃取过程中被大大削弱。
Abstract: The extraction and stripping characteristics of Versatic 10 and its mixtures with Cyanex 301 were investigated for the recovery of cobalt and nickel from sulfate solutions containing magnesium and calcium. The optimum extraction factors of Versatic 10 were initial pH of 2.5, O/A ratio of 1/3, and saponification rate of 60%. The loaded Versatic 10 was stripped by using 2 mol/L H2SO4 at O/A ratio of 1/1. The optimum volume ratio of Versatic 10 to Cyanex301 was 7/3 in the mixtures, which could effectively reduce the equilibrium pH to 3.50. And the loaded mixtures were stripped by using 2 mol/L H2SO4 at O/A ratio of 1/2. The reaction principle of Versatic10 and metal ions is an ion exchange reaction. However, in the extraction reaction of and metal ions, besides the ion exchange reaction, the strong coordination effect between Cyanex 301 and metal ions also exists, which is much weakened in the extraction reaction of the mixtures and metal ions.
Trans. Nonferrous Met. Soc. China 26(2016) 865-873
Qing-jun GUAN1, Wei SUN1, Gui-ying ZHOU2, Jia-peng LIU1, Zhi-gang YIN1
1. School of Mineral Processing and Bioengineering, Central South University, Changsha 410083, China;
2. National Engineering Laboratory of Biohydrometallurgy, Beijing General Research Institute for Non-ferrous Metals, Beijing 100088, China
Received 13 April 2015; accepted 29 September 2015
Abstract: The extraction and stripping characteristics of Versatic 10 and its mixtures with Cyanex 301 were investigated for the recovery of cobalt and nickel from sulfate solutions containing magnesium and calcium. The optimum extraction factors of Versatic 10 were initial pH of 2.5, O/A ratio of 1/3, and saponification rate of 60%. The loaded Versatic 10 was stripped by using 2 mol/L H2SO4 at O/A ratio of 1/1. The optimum volume ratio of Versatic 10 to Cyanex301 was 7/3 in the mixtures, which could effectively reduce the equilibrium pH to 3.50. And the loaded mixtures were stripped by using 2 mol/L H2SO4 at O/A ratio of 1/2. The reaction principle of Versatic10 and metal ions is an ion exchange reaction. However, in the extraction reaction of and metal ions, besides the ion exchange reaction, the strong coordination effect between Cyanex 301 and metal ions also exists, which is much weakened in the extraction reaction of the mixtures and metal ions.
Key words: solvent extraction; Versatic 10; Cyanex 301; nickel; cobalt; magnesium; calcium
1 Introduction
The increasing use of pressure acid leaching technology in the processing of lateritic nickel ores has created massive acidic leach liquors. After the removal of iron by chemical precipitation, besides valuable minerals like nickel and cobalt, the leach liquors tend to contain large numbers of other impurity ions, such as magnesium and calcium [1,2].
Much research has been carried out to separate nickel and cobalt from other metals using the solvent extraction [3-6]. Many researchers used Versatic 10 or its synergistic systems [7,8] for extraction of cobalt and nickel from leach liquors coming from oxidizing pressure leaching of mixed sulphide minerals. However, the equilibrium pH of those extraction systems was too high, which leads to excessive base consumption for pH adjustment.
CHENG and HOUCHIN [9] used the Versatic 10/decyl-4-pyridinecarboxylate ester synergistic system to separate nickel and cobalt from main impurities, such as manganese, magnesium, and calcium, and developed novel processes to directly recover nickel and cobalt from leach solutions without intermediate precipitation and re-leach. This system was successfully tested in batch and semi-continuous modes using pilot plant laterite leach solutions [10,11]. An almost complete separation of nickel, cobalt, copper and zinc from manganese, magnesium, calcium and chloride was achieved. However, the synergist, decyl-4- pyridinecarboxylate ester, is not commercially available, which makes the novel process difficult to commercialize in the nickel industry.
In the Goro process, a commercially available reagent, Cyanex 301, was used in the solvent extraction process [12-14]. The extractant was very effective in separating nickel and cobalt from manganese, magnesium and calcium in laterite leach solutions. Since the extractant was conducted at low pH, no base was required for pH adjustment. However, the stripping of nickel and cobalt has to be operated at high temperature using very strong acid [15,16]. Meanwhile, the stripping of the loaded organic phase of synergistic systems consisting of Cyanex 301 and other extractants was also difficult [17-19].
The present research work proposed a novel extraction system consisting of Versatic 10 and Cyanex 301 on selective extraction of nickel and cobalt in the presence of magnesium and calcium from sulfate solutions. The extraction system could not only reduce the equilibrium pH in the extraction process comparing with using Versatic 10 only, but also make the stripping of nickel and cobalt much easier comparing with using Cyanex 301 only. In this work, we first optimized the selectivity of Versatic 10 towards nickel and cobalt over magnesium and calcium in the sulphate solutions. And then on this basis the extraction and stripping characteristics of mixtures of Versatic 10 and Cyanex 301 were investigated.
2 Experimental
2.1 Aqueous and organic solutions
The synthetic feed solution was prepared by dissolving analytically pure metal sulfates in deionized water and contained 0.22 g/L Co2+, 1.9 g/L Ni2+, 0.39 g/L Ca2+ and 14.76 g/L Mg2+, which was the same as the real leach liquor. All reagents were produced by Sinopharm Chemical Reagent Co., Ltd., Shanghai, China.
Versatic 10 (shell chemical) used in this work is a synthetic tertiary carboxylic acid consisting of a mixture of highly branched isomers of C10 monocarboxylic acids, which has the following structure:

The relative molecular mass is 172 and the density (24 °C) is 0.91 g/cm3. The working pH of the Versatic 10 reagent is generally between 4 and 8. And the order of extraction of metal cations by Versatic 10 follows the order of hydrolysis constants of metal ions.
The extractant Cyanex 301, registered by Cyanamid Canada, was supplied by Cytec (Shanghai). The reactive component C16H34PS2H is the bis (2,4,4- trimethylpentyl)-dithio-phosphinic acid having the following structure:
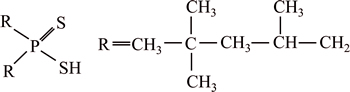
where R represents the 2,4,4-trimethylpentyl group. The relative molecular mass is 322 and the density (24 °C) is 0.95 g/cm3.
The organic diluent Mextral DT100 was obtained from Hallochem Pharma Co., Ltd.
2.2 Batch test procedures
All the tests were carried out at room temperature (25 °C). The initial pH of aqueous solutions was adjusted by small additions of sulfuric acid solutions, and the equilibrium pH was controlled by adjusting the initial pH in order to determine the metal extraction pH isotherms. The extractant Versatic 10 was saponified by 10 mol/L NaOH.
The organic phase and the aqueous phase were contacted at a certain O/A ratio in a 120 mL separation funnel. Then, the funnel was placed on an extraction purification shaker at 200 r/min for 4 min. The two phases were separated after equilibrating sufficiently on a steel stand. The equilibrium pH was measured using a Rex pH meter and the loaded organic solution was mixed with a selected scrub solution in order to remove the impurities in the loaded organic solution. After scrubbing, stripping of the loaded organic solution was carried out by sulfuric acid. The scrubbing and stripping were also carried out on the extraction purification shaker at the same speed for the same time in the extraction process.
For all experiments, the aqueous samples were analyzed by inductively coupled plasma atomic emission spectrometry (ICP-AES). The metal concentration in the loaded organic phase was deduced by subtracting the raffinate concentration from the initial metal concentration in the feed solution. The extractant and its extracted complexes were analyzed by Flourier translation infrared spectroscopy (FT-IR).
3 Results and discussion
3.1 Selectivity of Versatic 10 towards Ni and Co over Mg and Ca
3.1.1 Study of extraction process
In the process of extraction, the influence of three factors (pH, O/A ratio, saponification rate) on the extraction rates of nickel and cobalt was studied, compared with magnesium and calcium, by Versatic 10 and the optimum conditions were found.
1) Effect of pH on extraction
According to the general reaction of Versatic 10 and a divalent cation, such as Co2+ or Ni2+ [20]:
n(HA)2org+M2+aq [MA2nH2n-2]org+2H+aq (1)
[MA2nH2n-2]org+2H+aq (1)
The pH value has great influence on the solvent extraction reaction. Under the condition of other factors remaining the same, there is a one-to-one correspondence between the initial pH and the equilibrium pH, which can be inferred from Fig. 2.
As can be seen from Fig. 1, with the increase of pH, the extraction rates of nickel and cobalt rose significantly when the initial pH was less than 2.5, and then the growing trend became unobvious. However, the extraction rates of magnesium and calcium increased slightly with the increase of pH in the initial pH range of 1.7-2.0, and then remained almost unchanged. When the initial pH was higher than 2.5, the extraction rates of nickel and cobalt were both more than 85%, while those of magnesium and calcium were both less than 10%, achieving good extraction of nickel and cobalt and rejection of magnesium and calcium at the same time. Considering the characteristics of low pH of the leaching solutions, the optimum initial pH determined was 2.5, and the corresponding equilibrium pH was about 6.85 according to Fig. 2.
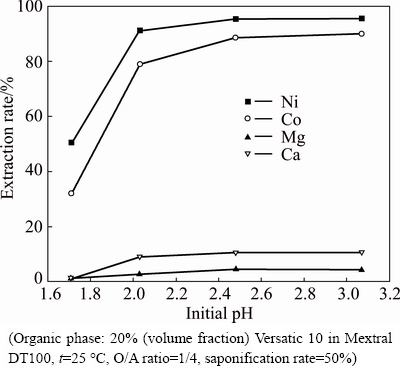
Fig. 1 Effect of initial pH on nickel and cobalt selectivity over magnesium and calcium in sulfate solutions with Versatic 10
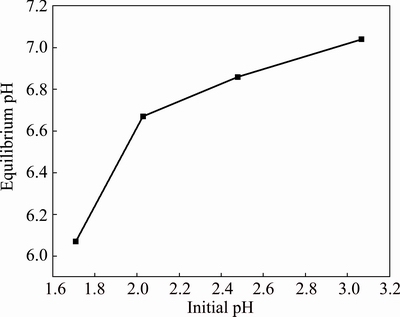
Fig. 2 Corresponding relationship between initial pH and equilibrium pH
2) Effect of O/A ratio on extraction
As shown in Fig. 3, with the decrease of O/A ratio, the extraction rates of nickel, cobalt, magnesium and calcium all suffered a decrease to some degree. This is mainly because, with the increase of the volume of the aqueous phase, the content of metal ions in the aqueous phase is bound to increase, but the loading capacity of the organic phase is fixed.
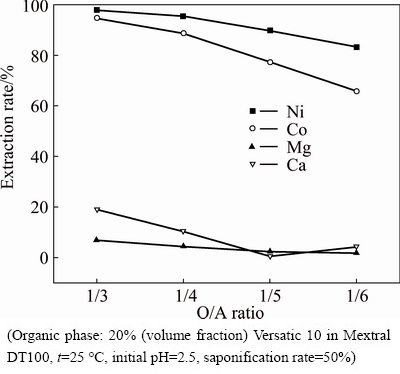
Fig. 3 Effect of O/A ratio on nickel and cobalt selectivity over magnesium and calcium in sulfate solutions with Versatic 10
When O/A ratio was 1/3, the extraction rates of nickel and cobalt were both more than 95%. Although at this point the extraction rate of calcium was a little high, the content of calcium in the aqueous phase was relatively low, which led to few amount of calcium extracted into the organic phase. At the same time, calcium was easily scrubbed out in the scrubbing process. Therefore, in order to recover valuable elements as completely as possible and achieve good separation of the valuable elements and the impurity elements, the optimum O/A ratio should be set at 1/3.
3) Effect of saponification rate on extraction
The results, presented in Fig. 4, show that with the increase of saponification rate, the extraction rates of nickel, cobalt and magnesium increased gradually; however, the extraction rate of calcium reached the maximum point at saponification rate of 50% before its gradual decrease.

Fig. 4 Effect of saponification rate on nickel and cobalt selectivity over magnesium and calcium in sulfate solutions with Versatic 10
According to the general reaction of Versatic 10 and a divalent cation (1), in the process of Versatic 10 saponification, H+ reacts with NaOH, and the reaction equilibrium moves to the right, which facilitates the extraction of metal ions and increases the extraction rate of metal ions. With the increase of saponification rare, the extraction rates of metal ions rise theoretically. The reason why the extraction rate of calcium decreases with the increase of saponification rate when the saponification rate is higher than 50% may be that the extraction speed of other metal ions rises faster than that of calcium with the increase of saponification rate, and the calcium extraction is suppressed.
When saponification rate was 60%, the extraction rates of nickel and cobalt were both higher than 96%. And then with the rise of saponification rate, the extraction rates of nickel and cobalt increased slightly, but the extraction rate of magnesium rose significantly. Due to high content of magnesium in the aqueous phase, as long as the extraction rate of magnesium has a little increase, a large amount of magnesium will be extracted into the organic phase. Therefore, the optimum saponification rate should be 60%.
Based on the above conditional experiments, the optimum conditions determined for the simultaneous extraction of nickel and cobalt from magnesium and calcium sulfate solutions in one stage were initial pH of 2.5, O/A ratio of 1/3, and saponification rate of 60%. And under the conditions, the extraction rates of nickel, cobalt, calcium and magnesium were 98.26%, 96.88%, 13.99% and 6.29% respectively, with the equilibrium pH of 6.82.
3.1.2 Study of scrubbing processes
The nickel- and cobalt-loaded organic phase, resulting from the extraction tests, was subjected to scrubbing with three types of scrub solutions under conditions of room temperature (25 °C), O/A ratio of 1/1, and 200 r/min for 4 min.
Table 1 shows that the efficiencies of the three scrub solutions were similar. In one stage almost all calcium and more than 70% magnesium were scrubbed out and nickel and cobalt were hardly affected.
With all factors considered, the deionized water was determined as the scrub solution, and the loaded organic phases were scrubbed twice with pure deionized water at O/A ratio of 1/1. The results are shown in Table 2.
3.1.3 Study of stripping processes
Factorial design and analysis of experiments were used in order to determine the optimum conditions in the stripping process. The variables and levels chosen to study the stripping of nickel and cobalt from the loaded organic phase by sulfuric acid solution are given in Table 3. Parameters with constant values throughout the design were room temperature (25 °C) and 200 r/min for 4 min.
It can be seen from Table 4 that high recoveries of nickel and cobalt stripped from the loaded organic phase were obtained. This proves the strippability of loaded Versatic 10 by H2SO4 solution. Based on the analysis of the results, the optimal conditions determined for the simultaneous stripping of nickel and cobalt from the loaded Versatic 10 in one stage were H2SO4 concentration of 2 mol/L and O/A ratio of 1/1.
3.2 Selectivity of mixtures of Versatic 10 and Cyanex 301 towards Ni and Co over Mg and Ca
As shown in the above experiments, nickel and cobalt could be effectively separated by the extractant Versatic 10 from the sulfate solution containing high contents of magnesium and calcium. However, the equilibrium pH was too high, which inevitably led to large amounts of alkali consumption and the difficulty in returning the raffinate to the dump.
Table 1 Metal ion concentrations in loaded organic solution and scrub solutions after scrubbing and their scrubbing efficiencies

Table 2 Metal ion concentrations in loaded organic solution and water after scrubbing and scrubbing efficiencies of deionized water

Table 3 Factors and variables

Table 4 Results of nickel and cobalt before or after stripping from loaded organic solution
In order to reduce the pH of the selective extraction of nickel and cobalt in the presence of magnesium and calcium effectively, the characteristics of the mixtures of Versatic 10 and Cyanex 301 were studied.
3.2.1 Effect of pH on extraction
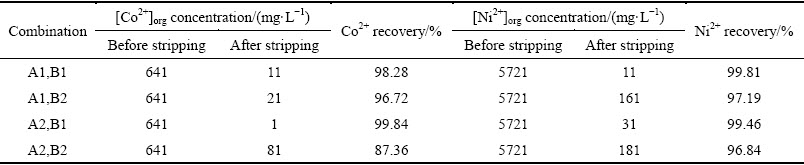
In order to determine the most appropriate volume ratio of Versatic 10 to Cyanex 301 in the mixtures to selectively extract nickel and cobalt in the presence of magnesium and calcium from the sulfate solution, extraction tests of the mixtures, which had different volume ratios, were carried out at a series of equilibrium pH. The results are shown in Figs. 5-9, respectively. Parameters were kept constant during experimentation, including room temperature (25 °C), extractant concentration of 20% (volume fraction), O/A ratio of 1/3, Versatic 10 saponification rate of 60% and 200 r/min for 4 min.
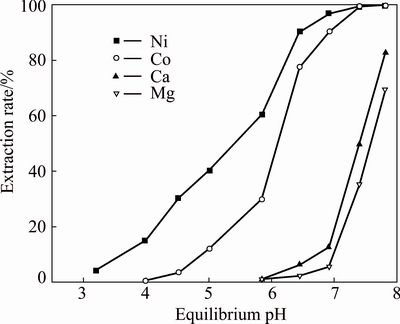
Fig. 5 Metal extraction pH isotherms with 20% Versatic 10 in Mextral DT100
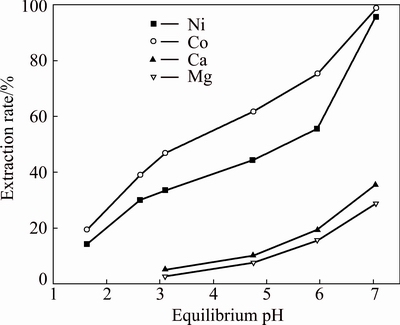
Fig. 6 Metal extraction pH isotherms with 20% (VVersatic 10: VCyanex 301= 8:2) Versatic 10 in Mextral DT100
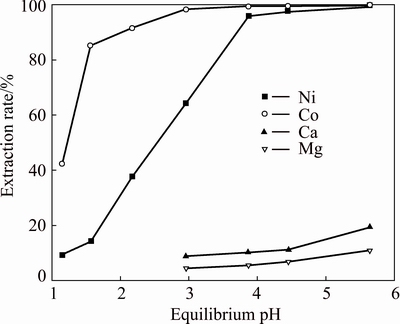
Fig. 7 Metal extraction pH isotherms with 20% Versatic 10 (VVersatic 10:VCyanex 301=7:3) in Mextral DT100
As can be seen from the above graphs, when the volume ratios of Versatic 10 to Cyanex 301 were 5:5, 6:4 and 7:3, the selectivity of nickel and cobalt over magnesium and calcium at low equilibrium pH (about 3.5) could be achieved. With the increase of Cyanex 301 in the Versatic 10-Cyanex 301 system, the extraction curves of nickel and cobalt moved to lower pH and the discrepancy between the curves of valuable ions and impurity ions increased, which represented the easier separation of valuable ions and impurity ions.

Fig. 8 Metal extraction pH isotherms with 20% Versatic 10 (VVersatic 10:VCyanex 301=6:4) in Mextral DT100
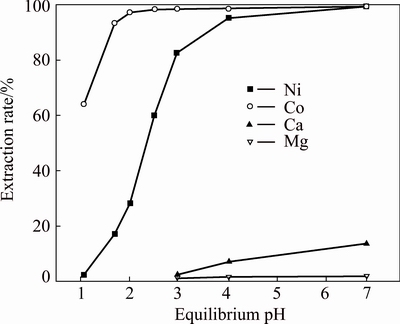
Fig. 9 Metal extraction pH isotherms with 20% Versatic 10 (VVersatic 10:VCyanex 301=5:5) in Mextral DT100
As shown in the above graphs and Table 5, relative to Versatic 10 system, the Versatic 10-Cyanex 301 system could make the cobalt extraction curve move to lower equilibrium pH significantly. When the volume ratios of Vesatic 10 to Cyanex 301 were 6:4 and 5:5, △pH50 was more than 5.1. For the nickel extraction curve, when the proportion of Cyanex 301 in the Versatic 10-Cyanex 301 system was small, the shift to the lower equilibrium pH was insignificant. However, with the increase of Cyanex 301 in the mixtures, the shift became larger. And When the volume ratio of Vesatic 10 to Cyanex 301 was 6:4, the shift of nickel extraction curve was the largest and △pH50 was 3.18. Therefore, in the extraction process, the optimum volume ratio of Versatic 10 to Cyanex 301 was 6:4.
3.2.2 Study of stripping process
In order to recover nickel and cobalt effectively in the loaded organic phase, the influence of the volume ratio of Versatic 10 to Cyanex 301, the concentration of H2SO4 and O/A ratio on the stripping process was studied.
Table 5 Comparison of pH50 and △pH50 of nickel and cobalt with Versatic 10 alone and mixtures of Versatic 10 and Cyanex 301

Figures 10 and 11 show that, with the increase of Versatic 10 in the Versatic 10-Cyanex 301 system, the stripping rates of nickel and cobalt increased significantly. Under the same condition, the stripping rate of nickel was higher than that of cobalt, and the sulfuric acid solution of 2.5 mol/L could get higher stripping rates than the sulfuric acid solution of 2 mol/L. When the volume ratio of Versatic 10 to Cyanex 301 was 7:3, the stripping rates of nickel and cobalt were more than 80%, and the increase of the stripping rates was insignificant as the concentration of the strip solution increased.
Therefore, according to the extraction and stripping processes, the optimum volume ratio of Versatic 10 to Cyanex 301 is set at 7:3 and 2 mol/L H2SO4 as the strip solution is appropriate.
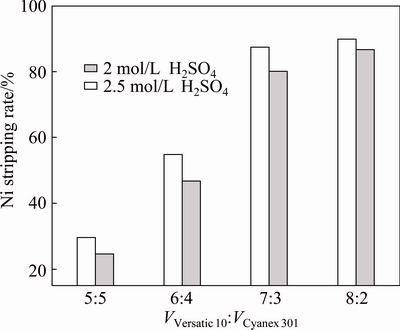
Fig. 10 Effect of volume ratio of Versatic 10 to Cyanex 301 and concentration of H2SO4 on nickel stripping rate at 25 °C and O/A ratio of 1/2
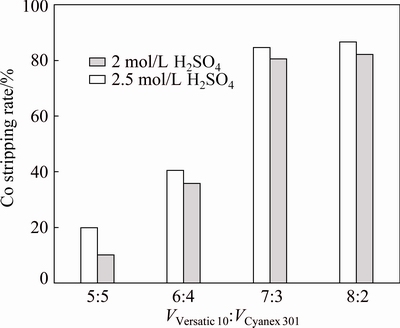
Fig. 11 Effect of volume ratio of Versatic 10 to Cyanex 301 and concentration of H2SO4 on cobalt stripping rate at 25 °C and O/A ratio of 1/2
As shown in Fig. 12, the stripping rates of nickel and cobalt grew as O/A ratio increased; however, when O/A ratio surpassed 1/2, the stripping rates had little increase. So, O/A ratio of 1/2 was appropriate.
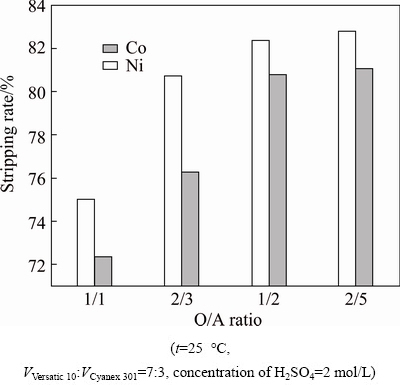
Fig. 12 Effect of O/A ratio on stripping process
3.3 FT-IR spectroscopy analysis
Figure 13 shows FT-IR spectra of the extractant Versatic 10, Versatic 10-Na complex, Versatic 10-Ni complex, and Versatic 10-Co complex. As can be seen from curve 1, in the high frequency region there is a very broad band (3400-3100 cm-1), owing to the overlap of C—H stretching vibration (3200-3100 cm-1) and a series of multiple overlapping peaks caused by O—H stretching vibration (3400-3200 cm-1) in hydrogen bonds formed in Versatic 10. However, there are no absorption bands at the same frequency in curves 2, 3 and 4, probably because the stretching vibration of O—H disappeared owing to the replacement of the hydrogen in hydroxyl group by metal ions.
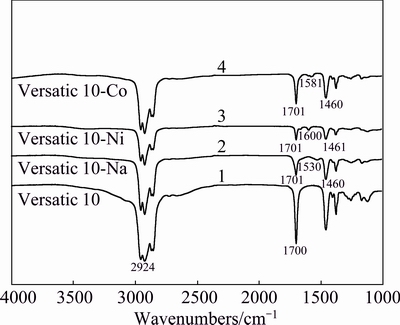
Fig. 13 FT-IR spectrums of Versatic 10 and its extracted complexes
The intensity of the C=O vibration at 1700 cm-1 in curve 1 is stronger than those in curves 2, 3 and 4. This is mainly because the hydrogen in —COOH group is replaced by metal ions and the metal ions have strong ability to attract electrons, which result in the transfer of electrons around the oxygen atom to the carbon atom in C=O group, weakening the charge difference between C and O and the change of the C=O dipole moment.
In the FT-IR spectra of metal carboxylic compounds, the frequency difference Δν (Δν=νas(COO-1)- νs(COO-1)) reflects the strength of the bond formed by the carboxylic oxygen and metals. Higher Δν values indicate more firm M—O bonds [21,22]. And when Δν<150 cm-1, the bond of M—O can be basically considered as the electrovalent bond [23]. As can be seen from Table 6, the bonds among Na, Ni, Co and O are all electrovalent bonds.
Table 6 Infrared frequencies of Versatic 10 and complexes of Versatic 10 and metal ions

Based on the above analysis, the reaction principle of the solvent extraction of Versatic 10 is an ion exchange reaction between the carboxylic hydrogen and the metal ion.
Figure 14 shows FT-IR spectra of the extractant Cyanex 301 (line 1), Cyanex 301-Ni complex (line 2), and Cyanex 301-Co complex (line 3). The S—H stretching peak of Cyanex 301 is at 2418 cm-1 in curve 1 and disappears both in curves 2 and 3 because of the Cyanex 301-Ni complex formation and the Cyanex 301-Co complex formation respectively, which indicates the replacement of the hydrogen in SH by the metal ions. The absorption peak of P=S at 617 cm-1 in curve 1 shifts to 601 cm-1 in curve 2 and 606 cm-1 in curve 3, showing that there is a strong coordination effect between the P=S bond in Cyanex 301 and the metal ions, which may be the reason why the loaded Cyanex 301 is hard to be stripped [24-26].

Fig. 14 FT-IR spectra of Cyanex 301 and its extracted complexes
Figure 15 shows FT-IR spectra of the mixtures (curve 1), mixtures-Ni complex (curve 2), and mixtures-Co complex (curve 3). Because Versatic 10 in the mixtures of Versatic 10 and Cyanex 301 was saponified by NaOH solution, the infrared absorption characteristic peaks of Versatic 10 complexes in curves 1, 2 and 3 are almost identical. The S—H stretching peak of Cyanex 301 at 2420 cm-1 in curve 1 disappears both in curves 2 and 3 because of the Cyanex 301-Ni complex formation and the Cyanex 301-Co complex formation respectively, which indicates the replacement of the hydrogen in SH by the metal ions. However, the absorption peak of P=S at 618 cm-1 in all curves has no obvious shifts, which indicates that the coordination effect between the P=S bond in Cyanex 301 and the metal ions is much weakened. And the reaction principle of the mixtures of Versatic 10 and Cyanex 301 is mainly an ion exchange reaction, which may be the reason why the stripping of the loaded mixtures is much easier than the loaded Cyanex 301.

Fig. 15 FT-IR spectra of mixtures (VVersatic 10:VCyanex 301=7:3) and its extracted complexes
4 Conclusions
1) Under the conditions of initial pH of 2.5, O/A ratio of 1/3, and saponification rate of 60%, Versatic 10 can extract nickel and cobalt by 98.26% and 96.88% respectively in one stage. And the equilibrium pH is 6.82. In the stripping test, the optimum conditions are 2 mol/L H2SO4 and O/A ratio 1/1. And the stripping rates of nickel and cobalt are 99.46% and 99.84%, respectively.
2) The optimum volume ratio of Versatic 10 to Cyanex 301 in the mixtures is 7:3, which can effectively reduce the equilibrium pH to 3.50 in the selective extraction of nickel and cobalt against magnesium and calcium. And the stripping rates of Ni and Co in loaded mixtures surpass 80% by using 2 mol/L H2SO4at O/A ratio of 1/2.
3) The reaction principle of Versatic 10 and metal ions is an ion exchange reaction. And in the extraction process of Cyanex 301, except for an ion exchange reaction, the strong coordination effect also exists between the P=S bond and metal ions. However, the main mechanism behind the reaction of the mixtures of Versatic 10 and Cyanex 301 is still mainly an ion exchange reaction and the coordination effect between the P=S bond in Cyanex 301 and metal ions is much weakened.
References
[1] GUO Xue-yi, SHI Wen-tang, LI Dong, TIAN Qing-hua. Leaching behavior of metals from limonitic laterite ore by high pressure acid leaching [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(1): 191-195.
[2] YU Guo-lin, ZHANG Ying, ZHENG Shi-li, ZOU Xing, WANG Xiao-hui, ZHANG Yi. Extraction of arsenic from arsenic-containing cobalt and nickel slag and preparation of arsenic-bearing compounds [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(6): 1918-1927.
[3] PRESTON J S, PREEZ A C D. Synergistic effects in the solvent extraction of some divalent metals by mixtures of Versatic 10 acid and pyridinecarboxylate esters [J]. Journal of Chemical Technology and Biotechnology, 1994, 61(2): 159-165.
[4] PRESTON J S, PREEZ A C D. Separation of nickel and calcium by solvent extraction using mixtures of carboxylic acids and alkylpyridines [J]. Hydrometallurgy, 2000, 58(3): 239-250.
[5] PREEZ A C D, PRESTON J S. Separation of nickel and cobalt from calcium, magnesium and manganese by solvent extraction with synergistic mixtures of carboxylic acids [J]. Journal of the South African Institute of Mining and Metallurgy, 2004, 104(6): 333-338.
[6] HAGHIGHI H K, MORADKHANI D, SALARIRAD M M. Separation of zinc from manganese, magnesium, calcium and cadmium using batch countercurrent extraction simulation followed by scrubbing and stripping [J]. Hydrometallurgy, 2015, 154: 9-16.
[7] CHENG C Y, URBANI M D, DAVIES M G. Recovery of nickel and cobalt from leach solutions of nickel laterites using a synergistic system consisting of Versatic 10 and Acorga CLX 50 [J]. Minerals Engineering, 2015, 77: 17-24.
[8] HUTTON-ASHKENNY M, BARNARD K R, IBANA D. The use of pyridine derivatives as accelerators in the solvent extraction of nickel from a nitrate matrix by LIX 63/VersaticTM 10 [J]. Hydrometallurgy, 2015, 153: 74-82.
63/VersaticTM 10 [J]. Hydrometallurgy, 2015, 153: 74-82.
[9] CHENG C Y, HOUCHIN M. Solvent extraction process for recovering nickel and cobalt from each solutions: U.S. Patent, 20040050212 [P]. 2004-3-18.
[10] CHENG Chu-yong. SX applications for nickel and cobalt: Pros and cons of existing processes and possible future developments [C]// Proceedings of ALTA SX/IX World Summit. Perth, Australia: ALTA Metallurgical Services, 2003: 131-143.
[11] CHENG C Y, URBANI M, HOUCHIN M. Manganese separation by solvent extraction in nickel laterite processing [C]// International Laterite Nickel Symposium-TMS 2004 Annual Meeting. Charlotte, North Carolina, USA: Wiley, 2004: 429-447.
[12] MIHAYLOV I, KRAUSE E, COLTON D F. The development of a novel hydrometallurgical process for nickel and cobalt recovery from Goro laterite ore [J]. CIM Bulletin, 2000, 93(1041): 124-130.
[13] MIHAYLOV I, KRAUSE E, LAUNDRY S W. Process for the extraction and separation of nickel and/or cobalt: U.S. Patent, 5378262 [P]. 1995-1-3.
[14] BACON G, MIHAYLOV I. Solvent extraction as an enabling technology in the nickel industry [J]. Journal of the South African Institute of Mining and Metallurgy, 2002, 102(8): 435-443.
[15] COTE G, BAUER D. Metal complexes with organothiophosphorus ligands and extraction phenomena [J]. Reviews in Inorganic Chemistry, 1989, 10(1-3): 121-144.
[16] GIBSON R W, RICE N M. A hydrochloric acid process for nickeliferous laterites [J]. Hydrometallurgy and Refining of Nickel and Cobalt, 1997, 1: 247-261.
[17] BATCHU N K, JEON H S, LEE M S. Solvent extraction of praseodymium (III) from chloride solutions by a mixture of Cyanex 301 and LIX 63 [J]. Journal of Industrial and Engineering Chemistry, 2014.
[18] BATCHU N K, SONU C H, LEE M S. Synergistic solvent extraction of manganese (II) with a mixture of Cyanex 272 and Cyanex 301 from chloride solutions [J]. Hydrometallurgy, 2013, 140: 89-94.
[19] BATCHU N K, SONU C H, LEE M S. Solvent extraction equilibrium and modeling studies of manganese from sulfate solutions by a mixture of Cyanex 301 and TBP [J]. Hydrometallurgy, 2014, 144: 1-6.
[20] ZHU Tun. Extraction and ion exchange [M]. Beijing: Metallurgical Industry Press, 2005. (in Chinese)
[21] NAKAMOTO K. Infrared spectra of inorganic and coordination compounds [M]. New York: John Wiley & Sons Inc, 1970.
[22] YANG Yen-sheng, GONG Meng-lian, LI Yuan-ying. Study on rare earth Versatic 10 [J]. Journal of Rare Earths, 1983, 1(2): 9-16.
[23] PENG An, DAI Zhen-rong. The proceedings of rare earth chemistry [M]. Beijing: Science Press, 1982. (in Chinese)
[24] STASZAK K, WIESZCZYCKA K, BURMISTRZAK P. Removal of cadmium (II) ions from chloride solutions by Cyanex 301 and Cyanex 302 [J]. Separation Science and Technology, 2011, 46(9): 1495-1502.
[25] RAJESH K J, RAMACHANDRA R B, JANARDHAN R K. Liquid-liquid extraction of tetravalent hafnium from acidic chloride solutions using bis (2, 4, 4-trimethylpentyl) dithiophosphinic acid (Cyanex 301) [J]. Separation Science and Technology, 2007, 42(4): 865-877.
[26] SHI Hua-qiang, FU Xun, ZHOU Xiao-dong, HU Zheng-shui. Preparation of organic fluid containing Ag nanoparticles with extractant Cyanex 301 [J]. Journal of Dispersion Science and Technology, 2005, 26(3): 315-319.
管青军1,孙 伟1,周桂英2,刘佳鹏1,殷志刚1
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 北京有色金属研究总院 生物冶金国家工程实验室,北京 100088
摘 要:考察萃取剂Versatic 10 以及它和Cyanex 301组成的复配萃取体系从含有镁钙离子的硫酸盐溶液中萃取分离镍钴离子的萃取和反萃特征。Versatic 10萃取分离镍钴和钙镁的最佳条件为初始pH值为2.5、油水体积比O/A为1/3、皂化率为60%。Versatic 10的负载有机相用2 mol/L的硫酸溶液以O/A为1/1进行反萃。复配萃取体系中Versatic 10和Cyanex 301的最佳体积比为7/3,复配体系能有效降低镍钴和钙镁萃取分离的平衡pH值至3.50,当O/A为1/2时,2 mol/L的硫酸溶液可以对负载有机相进行有效反萃。Versatic 10与金属离子进行萃取的反应机理为离子交换,而Cyanex 301与金属离子之间不仅有离子交换还有强烈的配位效应,这种配位效应在复配体系与金属离子萃取过程中被大大削弱。
关键词:溶剂萃取;Verrsatic 10;Cyanex 301;镍;钴;镁;钙
(Edited by Xiang-qun LI)
Foundation item: Project (2012BAB07B05) supported by the National Key Technology R&D Programs from the Ministry of Science and Technology of China; Project (B14034) supported by the Program of Introducing Talents of Discipline to Universities, China
Corresponding author: Wei SUN; Tel: +86-731-88830482; E-mail: sunmenghu@csu.edu.cn
DOI: 10.1016/S1003-6326(16)64178-X

