
Preparation and magnetic properties of nickel nanorods by thermal decomposition reducing methods
LUO Yu(罗 愉), ZHANG Jian-cheng(张建成), SHEN Yue(沈 悦),
JIANG Shu-tao(江树涛), LIU Guo-yong(刘国勇),WANG Lin-jun(王林军)
School of Materials Science and Engineering, Shanghai University, Shanghai 200072, China
Received 10 April 2006; accepted 25 April 2006
Abstract: The single-crystalline nickel nanorods with narrow size distribution and better magnetic properties were synthesized by thermal decomposition of nickel hydroxide nanorods precursor powders, which were produced by soft template method using nickel oxalic acid as raw material. The influences of hydrothermal reaction temperature and time on morphology of the products were investigated. The structure, morphology and magnetic properties of the products were characterized by X-ray powder diffraction (XRD), transmission electron microscopy (TEM), thermogravimetric differential scanning calorimetry (TGA-DSC) and vibrating sample magnetometer (VSM). The as-prepared nickel nanorods are uniform with a diameter of 10-15 nm and length 70-120 nm. The results of magnetic measurements show that the specific saturation magnetization(σs) and coercivity values(Hc) of the nickel nanorods are 50.649 A·m2/kg and 190.0×(103/4π)A/m,respectively. Finally, a possible mechanism for the formation of nickel nanorods was discussed briefly.
Key words: nickel hydroxide nanorods; nickel nanorods; PVP(polyvinylpyrrolidone); PEG(polyethylene glycol); specific saturation magnetization; coercivity
1 Introduction
Since 1991, progress in the synthesis and characterization of nanowire has been mainly driven by the need to understand the novel physical properties of one-dimensional nanoscale electronic and optoelectronic devices. One-dimensional nanostructure materials, such as nanowires, nanorods and nanotubes, have attracted special attention because of their potential magnetic, optical, electrical, and catalytic properties[1]. Magnetic nanorods represent an important family of magnetic nanostructures, still waiting for intense application due to the lack of understanding of their physical and chemical properties [2]. Nowadays, nickel nanorods, an important kind of ferromagnetic materials, have become a study focus of materials field in view of its extensive and potential application including high-density magnetic recording media, magnetic sensors and bio-molecular device etc. Recent years, many attempts have been made to synthesize one-dimensional nickel nanorods using a variety of nanofabrication techniques, such as hydrothermal microemulsion method [3], sonochemical and thermal decomposition of organic metal complexes [4], γ-ray irradiation[5], electrodeposition on alumina template[6-9] and soft template method [10-12]. However, the morphology control of one-dimensional nanostructure materials has been still a tremendous challenge.
Herein, we employ a simple and novel route by combining soft template and thermal decomposition methods for the preparation of nickel nanorods. The powders of single-crystalline nickel hydroxide nanorods are synthesized by a facile hydrothermal method using polyethylene glycol (PEG) and polyvinylpyrrolidone (PVP) as structure oriented agent and surface-modified reagent, respectively. Then, the single-crystalline nickel nanorods are synthesized by thermal decomposition method in the presence of H2 using Ni(OH)2 nanorods as the precursor. At the mean time, we investigate the magnetic properties of as-prepared nickel nanorods,The synthesis mechanism of nickel nanorods is also discussed and illustrated in this paper briefly.
2 Experimental
All the reagents were of analytical grade and were purchased from the local market and used without further purification. A typical synthesis route was performed as follows: An amount of 2 mmol H2C2O4 was dissolved in appropriate distilled water and dropped into the aqueous solution of NiCl2 at a stoichiometric Ni 2+/C2O42- molar ration of 1?1.1 during the magnetic stirring. The precipitates of NiC2O4·2H2O were filtered and dried in the air. Then, 1.2 g of NiC2O4·2H2O, 2 g PEG (the relative molecular mass is 20 000) and 0.808 g PVP were dissolved in 50 mL deionized water. After 30 min, 30 mL of 0.4 mol/L NaOH solution was added dropwise with stirring. The mixed slurry was sealed into a Teflon-lined stainless steel autoclaves and then heated at 160 ℃ for 12 h. After hydrothermal treatment, the precipitates of Ni(OH)2 was separated from solution by centrifugation, washed with deionized water and alcohol three times respectively, and dried in the air. Finally, green precursor powder was obtained. After that, the Ni(OH)2 precursor powders were reduced in the flow of H2 at 400 ℃ for 3 h so as to obtain the Ni nanorods. Other samples of nickel nanorods in this paper were prepared by the procedure similar to that for above sample, but under different hydrothermal conditions.
3 Results and discussion
Fig.1(a) shows the XRD pattern of Ni(OH)2 precursor obtained through hydrothermal processing. All the diffraction peaks can be indexed as hexagonal structure of Ni(OH)2 phase (space group: P3m1[164]) with lattice parameters a=0.312 6 nm,c=0.460 5 nm which is consistent with the reported data (JCPDS Card No. 2054-48-7). At the mean time a few impurity peaks are found which are speculated as characteristic peaks of remanent oxalates based on the 95%-98% yield of Ni(OH)2. The XRD pattern in Fig.1(b), corresponding to the thermally treated samples, shows the characteristic diffraction peaks of a face-centered cubic (fcc) structured Ni phase (space group Fm3m [225]). The lattice constant (a=0.352 5 nm) is in good agreement with the reported data (JCPDS Card No. 70-1849). The fact that no other impurity peaks are observed by XRD pattern indicates that Ni(OH)2 is completely decomposed to Ni at 400 ℃ for 3 h. Other samples in this paper prepared under different hydrothermal conditions have XRD patterns similar to Fig.1.
Fig.2 shows the typical TEM images of nickel nanorods prepared under the same hydrothermal temperature(160 ℃) and different hydrothermal reaction times. The resultant nickel crystallites initially come out
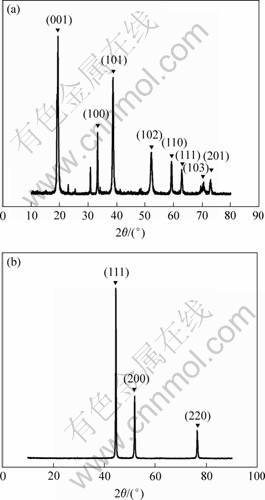
Fig.1 Typical XRD patterns of Ni(OH)2 nanorods synthesized with hydrothermal method(a) and Ni nanorods after thermal treatment(b) at 400 ℃ for 3 h
with sheet-like form as shown in Fig.2(a). And the sheets have irregularly shaped morphologies with sizes in the range of 35-45 nm. As depicted in Fig.2(b), with longer reaction time, some short rods are observed with some coexisting nanosheets, which are also observed by LI et al [10]. After reaction time of 12 h, nickel develops into homogeneous rod (Fig.2(c)) with diameter in the range of 10-15 nm and length 70-120 nm. And the corresponding selected area electron diffraction (SAED) pattern of Ni nanorods in Fig.2(c) indicates that they are single crystals. Compared Fig.2(d) with Fig.3(c), both the diameter and length of nickel nanorods do not change too much when the hydrothermal reaction time increases to 18 h.
Fig.3 shows the typical TEM images of nickel nanorods prepared under same hydrothermal time (16h) and different hydrothermal temperatures. Fig.3(a) shows that the products mainly take on sheet shape. The as-obtained Ni nanorods as shown in Fig.3(a) have

Fig.2 TEM images of samples prepared at same hydrothermal temperature (160 ℃) and different hydrothermal time: (a) 3 h; (b) 6 h ; (c) 12 h and its corresponding SAED pattern; (d) 18 h
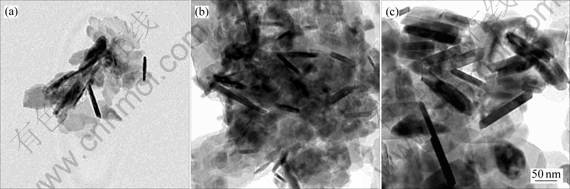
Fig.3 TEM images of samples prepared under same hydrothermal time (16h) and different hydrothermal temperatures: (a) 120 ℃; (b) 140 ℃; (c) 180 ℃
diameter of 10 nm and length of 50 nm, which are less than other samples of Fig.3. The image in Fig.3(b) shows that the obtained products are inhomogeneous nanorods with diameters of 10 nm and lengths of 70 nm. Compared Fig.3(c) with Fig.2(c), both the diameter and length of nickel nanorods have no obvious change.
It is worthwhile to notice that further elongation of nickel nanorods obtained in our experiment cannot occur when the hydrothermal reaction temperature is up to 160 ℃ and time is up to 12 h. This result is further proved by Figs.2(c) and (d) and Fig.3(c). It is likely that once the rods reach the length of polymer molecular chain, further growth of the particles can not occur. So the optimum synthesis hydrothermal temperature and time is 160℃ and 12 h, respectively. It is important for the synthesis of final nickel nanorods.
The thermal behavior of Ni(OH)2 nanorods is investigated with TGA and DSC measurements (Fig.4).
The TGA curve shows that Ni(OH)2 starts to volatilize (mass loss) at about 50 ℃. The mass loss happened slowly before 200 ℃ is attributed to the volatilization of water in the sample. The gradually mass loss of 6.5% between 200-300 ℃ ascribes to the loss of polymer (PEG, PVP). The major mass loss caused by the decomposition of Ni(OH)2 happens rapidly between 300 and 450 ℃ . The total mass loss is measured to be 32.5%. The DSC curve shows a maximum endothermic peak located at 319.6 ℃. The temperature range of the endothermic peak in the DSC curve fits well with that of mass loss in the TG curve, corresponding to the endothermic behavior during the decomposition of Ni(OH)2 to NiO.
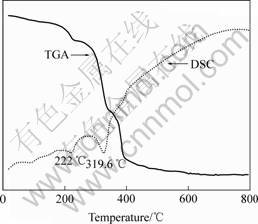
Fig.4 TG-DSC curves of Ni(OH)2 nanorods
We explored the possibility of using Ni(OH)2 nanorods as the precursor for synthesis of Ni nanorods. On the basis of TG and DSC results, we chose 400 ℃ to ensure the complete decomposition of Ni(OH)2.
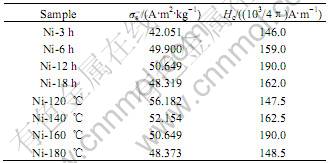
Fig.5 shows the magnetization curves of Ni-160 ℃(Ni-12 h) sample synthesized under hydrothermal tem- perature 160 ℃ and time 12 h. The corresponding specific saturation magnetization and coercive force (Hc) data of other samples are shown in Table 1. From Table 1, it is clear that the coercivity of Ni nanorods is increased with increasing of hydrothermal reaction temperature (before 160 ℃) and time (before 12 h). At the mean time, it is found that the coercivity of Ni nanorods decreases when the hydrothermal temperature and time exceed 160 ℃ and 12 h, respectively. It is well-known that reducing the nanorods diameter can increase the coercivity of nanorods [13]. Additionally, the coercivity of the nanorods can be enhanced by increasing the nanorods length, but it becomes saturation when the length exceeds a critical value at a certain size [14]. This phenomenon of coercive force is consistent with the results of TEM image. Our work demonstrated that the magnetic properties of Ni nanorods are closely related to their size and the growth conditions.
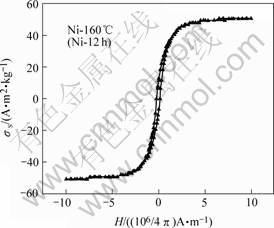
Fig.5 Magnetization curves of Ni-160(Ni-12h) sample
Table 1 Specific saturation magnetization(σs) and coercivity values(Hc) of nickel nanorods under different hydrothermal conditions
Based on the above experimental results, we discuss the mechanism of formation of nickel nanorods briefly. The PEG is very important in determining the morphology of the final product. PEG (H(OCH2—CH2)nOH) is a nonionic dispersant, —O— and —CH2CH2— serve as hydrophilic group and hydrophobic group respectively. Generally, PEG is a dentate long chain, but the dentate long chain changes to zigzag shape in aqueous solution (Fig.6) . The Ni2+ is bonded by oxygen atom of C—O—C chain in alkaline solution, so C2O42- is only substituted by OH- in the direction perpendicular to C—O—C—Ni2+ plane. At some relatively high temperature, C2O42- is gradually substituted by OH-, and in the end they form one-dimensional Ni(OH)2 nanorods structure. Since the role of PEG is to induce the OH- group to substitute along a certain direction, the length of PEG molecular chain should have some influence on the length of the nanorods. Further growth never occurs when the length of nanorods is up to the length of PEG molecular chain. Finally, nickel nanorods are obtained after thermal process of Ni(OH)2 nanorods powders. More detailed mechanism needs to further study and discuss in future work.
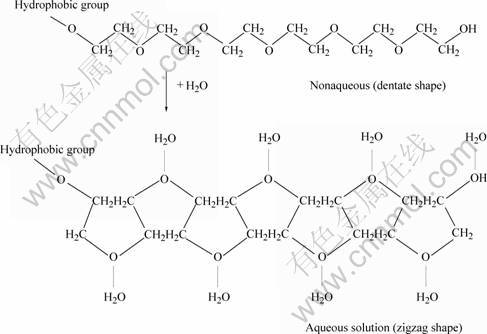
Fig.6 Change of PEG molecular chains in aqueous solution
4 Conclusions
The Ni nanorods with narrow size distribution and better magnetic properties were synthesized by a combined technique of soft template and thermal decomposition. The nanorods have a diameter of 10-15 nm and a length of 70-120 nm. This method is a new technique for controlled synthesis of metal nanorods, which has the advantages of both direct hydrothermal precipitate method and precursor thermal decomposition method. The final nanorod products have magnetic properties of σs=50.649A?m2/kg and 190.0×(103/4π) A/m.
References
[1] WU Z Y, LIU C M, GUO L, HU R, ABBAS M I, HU T D, XU H B. Structural characterization of nickel oxide nanowires by X-ray absorption near-edge structure spectroscopy [J]. J Phys Chem B, 2005, 109: 2512-2515.
[2] RAHMANA I Z, BOBOCA A, RAZEEB K M, RAHMAN M A. Analysis of magnetic interaction in Ni nanowire array grown using electrodeposition process [J]. Journal of Magnetism and Magnetic Materials, 2005, 290-291: 246-249.
[3] NI X M, SU X B, YANG Z P, ZHENG H G. The preparation of nickel nanorods in water-in-oil microemulsion [J]. J Cryst Growth, 2003, 252: 612-617.
[4] CORDENTE N, RESPAUD M, SENOCQ F, CASANOVE M J, AMIENS C, CHAUDRET B. Synthesis and magnetic properties of nickel nanorods [J]. Nano Lett, 2001(1): 565.
[5] WANG Feng,ZHANG Zhi-cheng, CHANG Zheng-qi. Effects of magnetic field on the morphology of nickel nanocrystals prepared by gamma- irradiation in aqueous solutions [J]. Materials Letters, 2002, 55: 27-29
[6] PAN Hui, LIU Bing-hai, YI Jia-bao, POH Cheekok, LIM Sanhua, DING Jun, FENG Yuan-ping, HUAN C H A, LIN Jian-yi. Growth of single-crystalline Ni and Co nanowires via electrochemical deposition and their magnetic properties [J]. J Phys Chem B, 2005, 109: 3094-3098.
[7] LIN S W, CHANG S C, LIU R S, HU S F, JAN N T. Fabrication and magnetic properties of nickel nanowires [J]. Journal of Magnetism and Magnetic Materials, 2004, 282: 28-31.
[8] CHIENA C L, SUN L, TANASE M, BAUER L A, HULTGREN A, SILEVITCH D M, MEYER G J, SEARSON P C, REICH D H. Electrodeposited magnetic nanowires: arrays, field-induced assembly, and surface functionalization [J]. Journal of Magnetism and Magnetic Materials, 2002, 249: 146-155.
[9] CHIRIAC H, MOGA A E, URSE M, OVARI T A. Preparation and magnetic properties of electrodeposited magnetic nanowires [J]. Sensors and Actuators A, 2003, 106: 348-351.
[10] WANG Qing-sheng, XU Zhu-de, YIN Hao-yong, NIE Qiu-lin. Fabrication of transition metal sulfides nanocrystallites via an ethylenediamine-assisted route [J]. Materials Chemistry and Physics, 2005, 90: 73-77.
[11] LI Xiao-lin, LIU Jun-feng, LI Ya-dong. Low-temperature conversion synthesis of M(OH)2 (M=Ni, Co, Fe) nanoflakes and nanorods [J]. Materials Chemistry and Physics, 2003, 80: 222-227.
[12] LIANG Zhen-hua, ZHU Ying-jie, HU Xian-luo. β-nickel hydroxide nanosheets and their thermal decomposition to nickel oxide nanosheets [J]. J Phys Chem B, 2004, 108: 3488-3491.
[13] NIELSCH K, WEHRSPOHN R B, BARTHEL J, KIRSCHNER J, GOSELE U, FICHER S F, KRONMULLER H. Hexagonally ordered 100 nm period nickel nanowire arrays [J]. Appl Phys Lett, 2001, 79: 1360.
[14] SKOMSKI R, ZENG H, ZHENG M, SELLMYER D. Magnetic localization in transition-metal nanowires [J]. J Phys Rev B, 2000, 65: 3900.
(Edited by YUAN Sai-qian)
Foundation item: Project(90206017) supported by the National Natural Science Foundation of China
Corresponding author: ZHANG Jian-cheng; Tel: +86-21-56331997; Fax: +86-21-56332475; E-mail:jchzhang@mail.shu.edu.cn