Cu2+-Ni2+-NH3-NH4+-C2O42--H2O体系金属离子沉淀行为热力学分析
来源期刊:中国有色金属学报(英文版)2021年第5期
论文作者:苗泽林 湛菁 徐子伟
文章页码:1475 - 1483
关键词:配位-沉淀平衡;热力学数学模型;Cu-Ni 草酸复盐;Cu-Ni合金
Key words:coordination-precipitation equilibrium; thermodynamic mathematical models; Cu-Ni oxalate complex salts; Cu-Ni alloy
摘 要:针对反应体系Cu2+-Ni2+-NH3-NH4+-C2O42--H2O中Cu2+和Ni2+的反应行为,根据物质守恒原理,建立热力学数学模型。模拟计算结果表明,该体系中金属离子的沉淀是一个复杂的动态平衡过程;在高pH条件下,Cu2+和Ni2+与NH3配合分别形成[Cu(NH3)n]2+ (n=3-5)和 [Ni(NH3)m]2+ (m=3-6)的反应占主导地位。当[C2O42-]T=0.6 mol/L时,Cu2+和Ni2+的共沉淀pH范围在[NH3]T为0.6 mol/L和4.2 mol/L时分别为2.0-6.5和2.0-5.5。在pH>7.0的纯水体系中,由于Cu2+和Ni2+的分步沉淀,Cu-Ni草酸复盐热分解后得到的产物为Cu-Ni复合物。改变沉淀介质为混合溶剂(水/乙醇)可最终制备具有高纯度和良好结晶度的棒状Cu-Ni合金粉末。
Abstract: To investigate the behaviors of Cu2+ and Ni2+ with the change of conditions in Cu2+-Ni2+-NH3-NH4+- C2O42--H2O reaction system, mathematical models of thermodynamics based on the principle of mass conservation were established. The simulation results indicate that the precipitation of metal ions from the aqueous phase is a complicated dynamic equilibrium process, during which the coordination reactions of Cu2+ and Ni2+ with NH3 forming [Cu(NH3)n]2+ (n=3-5) and [Ni(NH3)m]2+ (m=3-6) are predominant under high pH conditions, respectively. The pH ranges for the simultaneous precipitation of Cu2+ and Ni2+ are 2.0-6.5 and 2.0-5.5 when [NH3]T equals 0.6 and 4.2 mol/L, respectively, with the prefixed [C2O42-]T of 0.6 mol/L. Due to the fractional precipitation of Cu2+ and Ni2+, Cu-Ni composite is obtained after the thermal decomposition of Cu-Ni oxalate complex salts prepared in a pure water system when pH>7.0. By applying the mixed solvent (water/ethanol) as the precipitation medium, the Cu-Ni alloy rods can be finally fabricated with high purity and crystallinity.
Trans. Nonferrous Met. Soc. China 31(2021) 1475-1483
Ze-lin MIAO1, Jing ZHAN1,2, Zi-wei XU1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. National Engineering Laboratory for High Efficiency Recovery of Refractory Nonferrous Metals Resources,
Central South University, Changsha 410083, China
Received 5 June 2020; accepted 28 November 2020
Abstract: To investigate the behaviors of Cu2+ and Ni2+ with the change of conditions in Cu2+-Ni2+-NH3-NH4+- C2O42--H2O reaction system, mathematical models of thermodynamics based on the principle of mass conservation were established. The simulation results indicate that the precipitation of metal ions from the aqueous phase is a complicated dynamic equilibrium process, during which the coordination reactions of Cu2+ and Ni2+ with NH3 forming [Cu(NH3)n]2+ (n=3-5) and [Ni(NH3)m]2+ (m=3-6) are predominant under high pH conditions, respectively. The pH ranges for the simultaneous precipitation of Cu2+ and Ni2+ are 2.0-6.5 and 2.0-5.5 when [NH3]T equals 0.6 and 4.2 mol/L, respectively, with the prefixed [C2O42-]T of 0.6 mol/L. Due to the fractional precipitation of Cu2+ and Ni2+, Cu-Ni composite is obtained after the thermal decomposition of Cu-Ni oxalate complex salts prepared in a pure water system when pH>7.0. By applying the mixed solvent (water/ethanol) as the precipitation medium, the Cu-Ni alloy rods can be finally fabricated with high purity and crystallinity.
Key words: coordination-precipitation equilibrium; thermodynamic mathematical models; Cu-Ni oxalate complex salts; Cu-Ni alloy
1 Introduction
Expanding the fabrication methodologies for alloy materials has attracted substantially practical and theoretical research interest due to the significantly modified physical and chemical properties compared with their monometallic counterparts [1]. Benefiting from the intrinsically good magnetic, thermal-conductive, mechanical, and electronic properties [2-4], Cu-Ni alloy has earned great attention in the past few decades and was employed in the electromagnetic wave absorbing [5] and magnetocaloric fields [6], and also served as heterogeneous catalysts for many important reactions such as the evolution of hydrogen [7] and oxygen [8], decomposition of organic compounds [9,10], and the oxidation of alcohols [11,12]. Nickel has an identical face- centered-cubic (fcc) crystal structure and similar lattice parameters with copper (3.51 and 3.61  for Cu and Ni, respectively), making it possible to form the substitutional alloy by simply replacing the copper fraction with nickel atoms [13,14]. Currently, considerable efforts have been made to explore the fabrication approaches for Cu-Ni alloy, including mechanical alloying [6,15], direct-reduction [16], electrodeposition [17], spark discharge/laser ablation [18,19], hydrothermal [5,20], and ultra- sonically spray pyrolysis [21]. However, issues such as the relatively high cost, imprecise particle size and morphology control, extreme processing temperatures and usage of toxic organic solvents in some cases significantly restrict their further industrialization.
for Cu and Ni, respectively), making it possible to form the substitutional alloy by simply replacing the copper fraction with nickel atoms [13,14]. Currently, considerable efforts have been made to explore the fabrication approaches for Cu-Ni alloy, including mechanical alloying [6,15], direct-reduction [16], electrodeposition [17], spark discharge/laser ablation [18,19], hydrothermal [5,20], and ultra- sonically spray pyrolysis [21]. However, issues such as the relatively high cost, imprecise particle size and morphology control, extreme processing temperatures and usage of toxic organic solvents in some cases significantly restrict their further industrialization.
Liquid-phase precipitation, as a bottom-up strategy, combined with the post-annealing process at the moderate temperature could not only positively reduce the fabrication cost but also enable the rational tuning of metallic components at the near-atomic level and optimization of morphology and dimensions of the final products [22]. In our previous study, highly dispersed Cu and Ni powders were respectively fabricated through the thermal decomposition of the oxalate complex salts with a fibrous morphology synthesized in the corresponding liquid-phase system using oxalate acid as the precipitant and NH3 as the morphology controlling reagent [23,24]. These attempts could pave a new avenue for the large-scale fabrication of Cu-Ni alloy. Additionally, such NH3-induced one-dimensional (1D) morphology on the precursors coupled with the porous structure aroused by the removal of volatiles endows the metallic products with attractive properties such as the significantly increased specific surface area and the enhanced electronic conductivity [25,26]. However, due to the different thermodynamic properties of liquid-phase reactions involving Cu2+ and Ni2+ [27,28], their homogeneous precipitation and uniform distribution in oxalate complex salts would be the significant issues that affect the quality of the final products. Thus, a deep and comprehensive understanding of the behaviors of metal ions, i.e., Cu2+ and Ni2+, in this reaction system is urgently needed to evaluate their possibility of the simultaneous precipitation and to determine the corresponding pH range.
In the present study, the mathematical models of thermodynamics for the Cu2+-Ni2+-NH3-NH4+- C2O42--H2O reaction system will be established based on the mass conservation principle. With the change of pH, the total concentrations of Cu2+ and Ni2+ and their potential species in pure water solvent will be investigated. To achieve the maintenance of the predetermined molar ratio of metal ions in the raw materials at high pH conditions, the modification of the precipitation medium by using the water-ethanol mixture will be proposed to prepare the Cu-Ni oxalate complex salts and further thermal decomposition will be applied to obtaining Cu-Ni alloy powders with the accurate Ni to Cu molar ratio.
2 Mathematical models of coordination-precipitation equilibrium in Cu2+- Ni2+-NH3- NH4+-C2O42--H2O system
In the Cu2+-Ni2+-NH3-NH4+-C2O42--H2O reaction system, Cu2+ and Ni2+ could coordinate with various ligands including NH3, OH-, and C2O42- in the solution phase to form a series of metal complexes with different coordination numbers. Their cumulative formation constants (β) at 298 K [29] are listed in Table 1.
Table 1 Cumulative formation constants (β) of Cu/Ni complexes with different ligands (298 K)

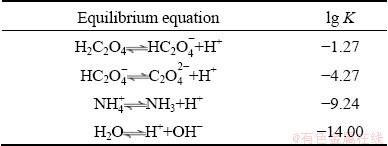
There also occurs the dissociation of weak acids and bases including H2C2O4, NH4+, and H2O, whose equilibrium constants (K) [29] are listed in Table 2.
Based on the mass balance, the mathematical models of the coordination-precipitation process in the Cu2+-Ni2+-NH3-NH4+-C2O42--H2O system are established, where [Me2+]T (Me=Cu, Ni), [NH3]T, [C2O42-]T represent the total concentration of metal ions, ammonia, and oxalate with all possible forms coexisting in the liquid phase, respectively.
Table 2 Dissociation reactions of acids and bases with corresponding equilibrium constants (K)
The total concentrations of metal ions [Me2+]T are calculated by the sum of the concentrations of free metal ions ([Cu2+] and [Ni2+]) and their three types of metal complexes:
[Cu2+]T=[Cu2+]+[Cu(NH3)n2+]T+[Cu(OH)n2-n]T+
[Cu(C2O4) n2-2n]T=[Cu2+]{1+104.31[NH3]+
107.98[NH3]2+1011.02[NH3]3+1013.32 [NH3]4+
1012.86[NH3]5+10pH-7.00+
102pH-14.32+103pH-25.00+104pH-37.5+
106.16[C2O42-]+108.50[C2O42-]2} (1)
[Ni2+]T=[Ni2+]+[Ni(NH3)m2+]T+[Ni(OH)m2-m]T+
[Ni(C2O4)m2-2m]T=[Ni2+]{1+102.80[NH3]+
105.04[NH3]2+106.77[NH3]3+107.96[NH3]4+
108.71 [NH3]5+108.74[NH3]6+10pH-9.03+
102pH-19.45+103pH-30.67+105.30[C2O42-]+
107.64[C2O42-]2+108.50[C2O42-]3} (2)
Meanwhile, [NH3]T and [C2O42-]T can be obtained according to the following equations:
[NH3]T=[NH3]+[NH4+]+∑n[Cu(NH3)n2+]+
∑m[Ni(NH3) m 2+]=[NH3](1+109.24-pH)+
[Cu2+]{104.31[NH3]+2×107.98[NH3]2+
3×1011.02 [NH3]3+4×1013.32[NH3]4+
5×1012.86 [NH3]5}+[Ni2+]{102.80[NH3]+
2×105.04[NH3]2+3×106.77[NH3]3+
4×107.96 [NH3]4+5×108.71 [NH3]5+
6×108.74[NH3]6} (3)
[C2O42-]T=[C2O42-]+[HC2O4-]+[H2C2O4]+
∑n[Cu(C2O4)n2-2n]+∑m[Ni(C2O4) m 2-2m]=
[C2O42-](1+104.27-pH+105.54-2pH)+
[Cu2+]{106.16[C2O42-]+2×108.50[C2O42-]2}+
[Ni2+]{105.30 [C2O42-]+2×107.64 [C2O42-]2+
3×108.50[C2O42-]3} (4)
Besides, Cu2+ and Ni2+ can form the precipitates with C2O42- and OH-, and their solubility product constants (Ksp) [29] are listed in Table 3. Therefore, the concentrations of free metal ions, namely [Cu2+] and [Ni2+], are determined by
[Cu2+]=min{10-7.64/[C2O42-], 10-19.66/[OH-]2} (5)
[Ni2+]=min{10-9.40/[C2O42-], 10-14.70/[OH-]2} (6)
Table 3 Solubility product constants (Ksp) of MeC2O4 and Me(OH)2 (Me=Cu, Ni)

3 Thermodynamic simulation results
According to the thermodynamic mathematical models established based on the reactions above, the values of [Cu2+]T, [Ni2+]T, and the concentrations of metal complexes with the change of pH can be calculated through Eqs. (1)-(6) with the constant values of [NH3]T and [C2O42-]T.
3.1 Relationship between lg[Cu2+]T and pH
The curves of lg[Cu2+]T vs pH in Cu2+-Ni2+- NH3-NH4+-C2O42--H2O reaction system with [NH3]T ranging from 0.6 to 4.2 mol/L are plotted in Fig. 1. The [C2O42-]T is predefined to be 0.6 mol/L. It can be observed that when pH<4.0, these curves overlap and show the same declining trends with the increase of pH. Combined with Fig. 2 that displays the concentrations of various copper species at different pH values, the dominance of [Cu(C2O4)n]2-2n complex indicates the interaction between Cu2+ and C2O42- and the negligible influence of NH3 in pH range of 0-4.0. However, when pH=4.0-9.0, the lg[Cu2+]T dramatically increases, which can be ascribed to the prominent coordination effect of NH3. It can also be noticed from Fig. 2 that [Cu(NH3)n]2+ gradually increases at this time, especially for those copper ammonia complexes with the higher coordination numbers (n=3-5), parallel to the continuous decrease of free Cu2+. At the higher pH values (pH>10.0), Cu2+ is more likely to be precipitated as Cu(OH)2, leading to a decrease of lg[Cu2+]T again. When pH is larger than 13.0, the total concentrations of [Cu(OH)n]2-n complexes will exceed those of other species and are predominant in the system. Meanwhile, the coordination reaction between Cu2+ and OH- results in a slight increase of [Cu2+]T (pH>12.0) as shown in Fig. 1.

Fig. 1 Relationship between lg[Cu2+]T and pH with different [NH3]T ([C2O42-]T=0.6 mol/L)
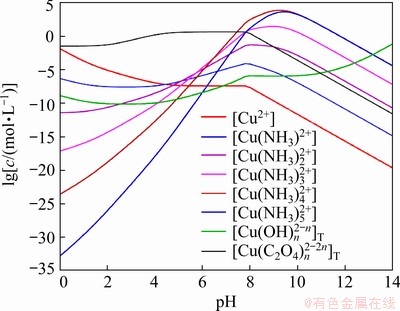
Fig. 2 Concentrations of various Cu2+ species in Cu2+-Ni2+-NH3-NH4+-C2O42--H2O system ([C2O42-]T= 0.6 mol/L, [NH3]T=3.0 mol/L)
3.2 Relationship between lg[Ni2+]T and pH
Figure 3 shows the relationships between lg[Ni2+]T and pH with the predefined [C2O42-]T of 0.6 mol/L and different [NH3]T, and Fig. 4 displays the corresponding change of concentration of Ni2+ species coexisting in Cu2+-Ni2+-NH3-NH4+- C2O42--H2O reaction system. The total concentration of Ni2+ decreases initially at the low pH values due to the formation of NiC2O4. This kind of oxalate salt was reported to be likely dissolved in the extremely acidic conditions [30], resulting in the highest concentration of free Ni2+ at pH=0, as shown in Fig. 4. It can also be found that [Ni(C2O4)m]2-2m complexes are the main species of nickel ions in the pH range of 0-8.5. With the continuous increase of pH, the capability of Ni2+ coordinating with NH3 gradually becomes prominent and even more significant than that with C2O42- when pH>9.0, leading to the sharp increase of lg[Ni2+]T (Fig. 3). Nickel ammonia complexes turn to be dominant at this point, especially [Ni(NH3)m]2+ (m=3-6). Similarly, due to the formation of Ni(OH)2, lg[Ni2+]T decreases when pH is larger than 11.0. However, in Fig. 3, the increase of lg[Ni2+] when pH>12.0 can only be observed at the low values of [NH3]T (0.6 and 1.2 mol/L), which is slightly different from the behaviors of copper ions in the same reaction system.

Fig. 3 Relationship between lg[Ni2+]T and pH with different [NH3]T ([C2O42-]T = 0.6 mol/L)
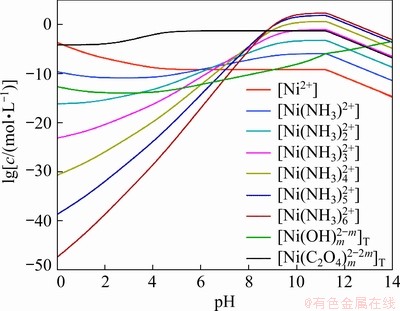
Fig. 4 Concentrations of various Ni2+ species in Cu2+-Ni2+-NH3-NH4+-C2O42--H2O system ([C2O42-]T= 0.6 mol/L, [NH3]T=3.0 mol/L)
3.3 Coprecipitation pH ranges of Cu2+ and Ni2+
According to the above thermodynamic simulation results, it can be concluded that the precipitation of Cu2+ and Ni2+ into oxalate complex salts is a complicated dynamic equilibrium process associated with the competition of various reactions in the system, during which the precipitating rates and precipitation sequences of metal ions are significantly influenced by their interactions with NH3/NH4+, OH-, and C2O42-, etc. To determine the coprecipitating pH ranges, the curves of lg[Me2+] (Me=Cu, Ni) vs pH with the minimum and maximum [NH3]T (0.6 and 4.2 mol/L) and the fixed [C2O42-]T of 0.6 mol/L are plotted in Fig. 5. Due to the larger cumulative formation constants (β) of copper complexes compared to those of nickel species, the total concentration of Cu2+ in solution is constantly higher than that of Ni2+ until pH reaches 11.0. The simultaneous precipitation of metal ions and Cu/Ni molar ratio control could only be achieved at pH=2.0-5.5 and 2.0-6.5 for [NH3]T= 4.2 mol/L and 0.6 mol/L, respectively, where both of [Cu2+]T and [Ni2+]T are lower than 10-5 mol/L. However, it should be noted that the fibrous morphology cannot be formed in this pH range based on our previous reports [22-24]. When pH>7.0, the coprecipitation of Ni2+ and Cu2+ with oxalate ions would be thermodynamically unfavorable to control, leading to the change of the predefined molar ratio of Cu/Ni in the precipitates and the high possibility of the formation of Cu-Ni composites as the final products instead of the substitutional alloys after the annealing process. Therefore, to obtain Cu-Ni alloy with the maintained metallic molar ratio and the fibrous morphology, a modified solvent of the water-ethanol mixture will be applied due to the reduced solubility of oxalate precipitates and metal complexes in a solvent with a lower surface tension than water, and also its successfully practical utilization in the fabrication of Ni-Co fibrous alloy powders [22].
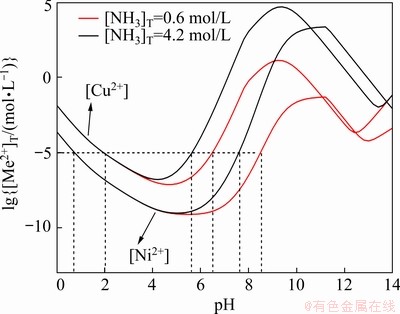
Fig. 5 Coprecipitation pH ranges of Cu2+ and Ni2+ with [NH3]T =0.6 and 4.2 mol/L ([C2O42-]T=0.6 mol/L)
4 Preparation of Cu-Ni alloy powders
4.1 Experimental and characterization
All of the reagents including nickel(II) chloride hexahydrate (NiCl2·6H2O), copper(II) chloride dihydrate (CuCl2·2H2O), oxalic acid dihydrate (H2C2O4·2H2O), ammonium hydroxide (NH3·H2O), polyvinylpyrrolidone (PVP), and ethanol were of analytical grade and directly used without further purification.
In a typical experiment, a 100 mL solution dissolving CuCl2·2H2O (7.842 g), NiCl2·6H2O (9.702 g) and the surfactant PVP (0.5 g) was slowly dripped into 100 mL H2C2O4 (13.010 g) solution at the rate of 2.22 mL/min, during which the pH was adjusted to 7.6 by the dropwise addition of NH3·H2O. The system was maintained at a constant temperature of 323 K in a water bath and vigorously stirred for 2 h after feeding. Precipitated solids were collected, rinsed by deionized water and ethanol, and dried in the vacuum oven at 353 K for 12 h. The pure water solvent was replaced by a mixture of water and ethanol (1/1, volume ratio) as the modified system. Final products were obtained via thermal decomposition of the prepared oxalate complex salts in Ar/H2 (95/5, volume ratio) at 673 K for 30 min.
Field-emission scanning electron microscopy (FE-SEM, TESCAN MIRA3 LMU) and transmission electron microscopy (TEM, Tecnai G2 20ST) images were obtained to show the morphology and particle sizes of the prepared powders. Energy-dispersive X-ray spectroscopy (EDX, GENESIS 60S) measurement was performed to detect the molar ratio of Cu to Ni at the selected area of the oxalate complex salts. X-ray diffractometer (XRD, Rigaku-TTR III) with the radiation of Cu Kα (λ=0.154056 nm) was applied to determining the phase and composition of the final products.
4.2 Results and discussion
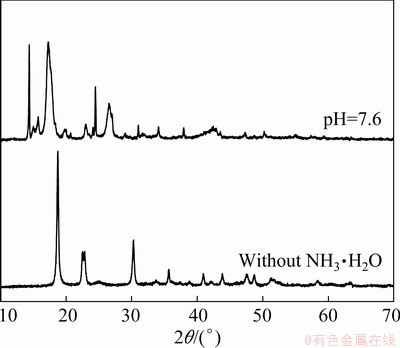
Fig. 6 XRD patterns of Cu-Ni oxalate complex salts prepared at pH=7.6 and without NH3·H2O in modified solvent system [32]
It has been reported that the morphology and particle size of oxalate complex salts can be simply controlled by adjusting the volume of NH3·H2O added into the suspension, and the quasi-1D morphology will be formed at high pH values due to the shape-induced effect of NH3, for example, 8.0-8.2 for Cu [23], 8.4-8.8 for Ni [24], 8.0-8.4 for Ni-Co [22], and 8.0 for Co-Zn system [31], etc. Thus, the alkaline environment of the Cu-Ni system is expected to be favorable for the precipitation of Cu-Ni alloy precursors with the aforementioned 1D morphology. The XRD patterns of Cu-Ni oxalate complex salts prepared at pH=7.6 and without NH3·H2O are both recorded and displayed in Fig. 6. The Cu-Ni alloy precursors obtained at pH=7.6 is suggested to be a novel solid solution containing NH3 instead of the mechanical mixture of MeC2O4 (Me=Cu and Ni), which has been illustrated in detail according to the recently published work [32].
Figure 7(a) shows the SEM images of Cu-Ni oxalate complex salts obtained at pH=7.6 in the modified reaction system. It can be observed that the prepared precursors display a fibrous morphology with a length of 10-20 μm and a diameter of 0.4-0.6 μm. The EDX results (Fig. 7(b)) indicate the equivalent atomic proportion of Cu and Ni in the precipitates, which is the same as the molar ratio of metal sources in the feedstock. By releasing the volatile components including water of crystallization, coordinated NH3, and CO2 originated from the thermal decomposition of oxalate salts, a special porous Cu-Ni alloy powders was formed (Fig. 7(c)), which can be clearly observed from the TEM image (Fig. 7(d)) by showing the numerous interconnected nanoparticles with the existence of pores at the nanoscales.
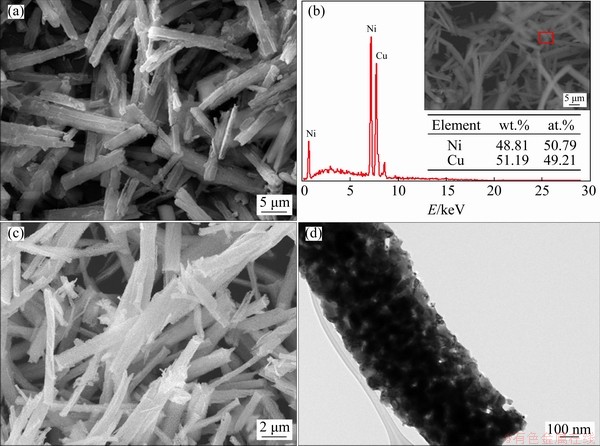
Fig. 7 SEM image (a) and EDX result (b) of Cu-Ni oxalate complex salts prepared in modified system, SEM (c) and TEM (d) images of corresponding final products
The crystal phase and composition of final products obtained by thermal decomposition of precursors prepared in the pure water and mixed solvent system were identified and compared by the XRD technique. The diffraction patterns indicate that the product obtained from the pure water system (Fig. 8(a)) has the mixed phases of Cu and Ni. The diffraction peaks located at 2θ=43.3°, 50.4°, and 74.1° are ascribed to the (111), (200) and (220) crystal facets of the fcc structured Cu (JCPDS No.04-0836) [33,34] and the peaks at 2θ of 44.5°, 51.8°, and 76.4°correspond to the (111), (200), and (220) facets of Ni (JCPDS No. 04-0850) [35]. However, when using a ethanol-H2O mixture as the solvent, Cu-Ni alloy was finally obtained (Fig. 8(b)), and the three diffraction peaks at 2θ=43.9°, 51.2°, and 75.3° can be well-indexed to the (111), (200), and (220) facets of Cu-Ni alloy (JCPDS No. 65-9047). No peaks of Cu, Ni, or any other substances were detected, indicating its high purity and thus ruling out the fractional precipitation of Cu2+ and Ni2+ during the coordination-coprecipitation process.
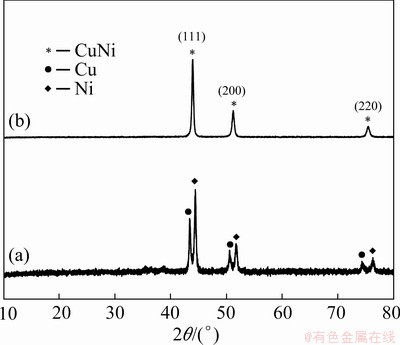
Fig. 8 XRD patterns of Cu-Ni composite (a) and Cu-Ni alloy (b)
5 Conclusions
(1) The thermodynamic mathematical models of Cu2+-Ni2+-NH3-NH4+-C2O42--H2O system were established according to the mass conservation principle, and the curves of lg[Me2+] (Me=Cu, Ni) vs pH were plotted to illustrate the trends of concentration of metal ions with the change of pH during the precipitation process.
(2) At the low pH values, free Me2+ and Cu(C2O4)n2-2n are predominant during which the precipitation of Me2+ by C2O42- mainly occurs. While at high pH conditions, the dominance of [Cu(NH3)n]2+ (n=3-5) and [Ni(NH3)m]2+ (m=3-6) in solution indicates the preferable coordination of metal ions with NH3 than other ligands. With the predefined [C2O42-]T of 0.6 mol/L, the coprecipitation pH ranges of Cu2+ and Ni2+ with [NH3]T of 0.6 mol/L and 4.2 mol/L are 2.0-6.5 and 2.0-5.5, respectively.
(3) Experimental results confirmed the thermodynamically favorable fractional precipitation of Cu2+ and Ni2+ when preparing the oxalate complex salts at pH=7.6, leading to the formation of Cu-Ni composite after the annealing process. By applying the water-ethanol mixture as the modified precipitation medium, Cu-Ni alloy powders with a quasi-1D morphology and the porous structure were finally obtained.
Acknowledgments
The authors are grateful for the financial supports from Natural Science Foundation of Hunan Province, China (No. 2020JJ4735), Science and Technology Department of Hunan Province Tackling Key Scientific and Technological Problems and Transformation of Major Scientific and Technological Achievements, China (No. 2018GK4001), and the Hunan Key Laboratory for Rare Earth Functional Materials, China (No. 2017TP1031).
References
[1] YEN H, SEO Y, KALIAGUINE S, KLEITZ F. Role of metal-support interactions, particle size, and metal-metal synergy in CuNi nanocatalysts for H2 generation [J]. ACS Catalysis, 2015, 5: 5505-5511.
[2] QUINTANA A, MENENDEZ E, ISARAIN-CHAVEZ E, FORNELL J, SOLSONA P, FAUTH F, BARO M D, NOGUES J, PELLICER E, SORT J. Tunable magnetism in nanoporous CuNi alloys by reversible voltage-driven element-selective redox processes [J]. Small, 2018, 14: 1704396.
[3] CHUNG S W, YOON T J, NOH J S, KANG C Y. Microstructure and mechanical properties in the friction stir welded C70600 alloy [J]. Journal of Welding and Joining, 2018, 36: 60-66.
[4] SUN Jing, CHEN Yan-ru, HUANG Ke-ke, LI Kai, WANG Qin. Interfacial electronic structure and electrocatalytic performance modulation in Cu0.81Ni0.19 nanoflowers by heteroatom doping engineering using ionic liquid dopant [J]. Applied Surface Science, 2020, 500: 144052.
[5] ZHAO Biao, ZHAO Wan-yu, SHAO Gang, FAN Bing-bing, ZHANG Rui. Morphology-control synthesis of a core-shell structured NiCu alloy with tunable electromagnetic-wave absorption capabilities [J]. ACS Applied Materials & Interfaces, 2015, 7: 12951-12960.
[6] ALLEG S, SOUILAH S, DADDA K, SUNOL J J, HLIL E K, LASSRI H. Investigation of the critical behavior and magnetocaloric properties in the nanocrystalline CuNi powders [J]. Journal of Magnetism and Magnetic Materials, 2017, 444: 54-60.
[7] SHEN Yi, ZHOU Yong-fang, WANG Duo, WU Xi, LI Jia, XI Jing-yu. Nickel-copper alloy encapsulated in graphitic carbon shells as electrocatalysts for hydrogen evolution reaction [J]. Advanced Energy Materials, 2018, 8: 1701759.
[8] KUMAR M, JEONG D I, YOON D H. Copper nickel alloy nanorods textured nanoparticles for oxygen evolution reaction [J]. Electrochimica Acta, 2020, 333: 135545.
[9] RUDNEV A V, LYSAKOVA A S, PLYUSNIN P E, BAUMAN Y I, SHUBIN Y V, MISHAKOV I V, VEDYAGIN A A, BUYANOV R A. Ni-Cu and Ni-Co alloys: Synthesis, structure, and catalytic activity for the decomposition of chlorinated hydrocarbons [J]. Inorganic Materials, 2014, 50: 566-571.
[10] SHEN Yi, LUA A C. Polyol synthesis of nickel-copper based catalysts for hydrogen production by methane decomposition [J]. International Journal of Hydrogen Energy, 2015, 40: 311-321.
[11] AN Ya-jing, IJAZ H, HUANG Ming, QU Jian-qing, HU Shi. The one-pot synthesis of CuNi nanoparticles with a Ni-rich surface for the electrocatalytic methanol oxidation reaction [J]. Dalton Transactions, 2020, 49: 1646-1651.
[12] TIAN Xi-ke, ZHAO Xiao-yu, ZHANG Li-de, YANG Chao, PI Zhen-bang, ZAHNG SU-xin. Performance of ethanol electro-oxidation on Ni-Cu alloy nanowires through composition modulation [J]. Nanotechnology, 2008, 19, 215711.
[13] NAGHASH A R, ETSELL T H, XU S. XRD and XPS study of Cu-Ni interactions on reduced copper-nickel-aluminum oxide solid solution catalysts [J]. Chemistry of Materials, 2006, 18: 2480-2488.
[14] YU Chao, FU Jia-ju, MUZZIO M, SHEN Tun-li, SU Dong, ZHU Jun-jie, SUN Shou-heng. CuNi nanoparticles assembled on graphene for catalytic methanolysis of ammonia borane and hydrogenation of nitro/nitrile compounds [J]. Chemistry of Materials, 2017, 29: 1413-1418.
[15] BAN I, STERGAR J. DROFENIK M, FERK G, MAKOVEC D. Synthesis of copper-nickel nanoparticles prepared by mechanical milling for use in magnetic hyperthermia [J]. Journal of Magnetism and Magnetic Materials, 2011, 323: 2254-2258.
[16] WANG Meng-lin, WANG Liang-bing, LI Hong-liang, DU Wen-peng, KHAN M U, ZHAO Song-tao, MA Chao, LI Zhen-yu, ZENG Jie. Ratio-controlled synthesis of CuNi octahedra and nanocubes with enhanced catalytic activity [J]. Journal of the American Chemical Society, 2015, 137: 14027-14030.
[17] GAO M Y, YANG C, ZHANG Q B, YU Y W, HUA Y X, LI Y, DONG P. Electrochemical fabrication of porous Ni-Cu alloy nanosheets with high catalytic activity for hydrogen evolution [J]. Electrochimica Acta, 2016, 215: 609-616.
[18] MUNTEAN A, WAGNER M, MEYER J, SEIPENBUSCH M. Generation of copper, nickel, and CuNi alloy nanoparticles by spark discharge [J]. Journal of Nanoparticle Research, 2016, 18: 229
[19] LIN Zhao-yong, LI Ji-ling, LI Li-hua, YU Li-li, LI Wei-jia, YANG Guo-wei. Manipulating the hydrogen evolution pathway on composition-tunable CuNi nanoalloys [J]. Journal of Materials Chemistry A, 2017, 5: 773-781.
[20] WU Song-ping, NI Jing, JIAO Li, ZENG Zhen-ou. Preparation of ultra-fine copper-nickel bimetallic powders with hydrothermal-reduction method [J]. Materials Chemistry and Physics, 2007, 105: 71-75.
[21] APAYDIN R O, EBIN B, GüRMEN S. Single-step production of nanostructured copper-nickel (CuNi) and copper-nickel-indium (CuNiIn) alloy particles [J]. Metallurgical and Materials Transaction A, 2016, 47: 3744-3752.
[22] ZHAN Jing, HE Yue-hui, ZHOU Di-fei, ZHANG Chuan-fu. Thermodynamic analysis on synthesis of fibrous Ni-Co alloys precursor and Ni/Co ratio control [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 1141-1148.
[23] FAN You-qi, ZHANG Chuan-fu, WU Jian-hui, ZAHN Jing, YANG ping. Composition and morphology of complicated copper oxalate powder [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 165-170.
[24] WU Jian-hui, LIU Gang, SU Tao, ZHANG Wen-hong, LUO Mei-mei, WEI Tao. Preparation of fibrous nickel powder by precipitation transformation coupled with thermal decomposition [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 2653-2660.
[25] ZHAN Jing, YAO Yong-lin, ZHANG Chuan-fu, LI Chang- jun. Synthesis and microwave absorbing properties of quasione-dimensional mesoporous NiCo2O4 nanostructure [J]. Journal of Alloys and Compounds, 2014, 585: 240-244.
[26] HUO Da, KIM M J, LYU Zhi-heng, SHI Yi-feng, WILEY B J, XIA You-nan. One-dimensional metal nanostructures: From colloidal syntheses to applications [J]. Chemical Reviews, 2019, 119: 8972-9073.
[27] FAN You-qi, ZAHNG Chuan-fu, ZHAN Jing, WU Jian-hui. Thermodynamic equilibrium calculation on preparation of copper oxalate precursor powder [J]. Transactions of Nonferrous Metals Society of China, 2008, 18: 454-458.
[28] ZAHN Jing, ZHANG Chuan-fu, LI Tie-jing, WU Jian-hui. Thermodynamic analysis on preparation of fibrous NiO precursor powders with oxalate precipitation process [J]. Transactions of Nonferrous Metals Society of China, 2005, 15: 926-930.
[29] DEAN J A. Lange’s handbook of chemistry [M]. 14th ed. New York, London: McGraw-Hill, Inc., 1999.
[30] ZHANG Chuan-fu, YAO Yong-lin, ZHAN Jing. Thermo- dynamics of precipitation-coordination equilibrium in Fe2+-Ni2+-NH3-NH4+-C2O42--H2O system [J]. The Chinese Journal of Nonferrous Metals, 2012, 22: 2938-2943. (in Chinese)
[31] CHEN Jun, ZHAN Jing, LU Er-ju, WAN Yu-chi, JIN Zhen, QI Hao-zhi. Facile template-free fabrication of mesoporous ZnCo2O4 fibers with enhanced photocatalytic activity under visible-light irradiation [J]. Materials Letters, 2018, 220: 66-69.
[32] MIAO Ze-lin, XU Chang-fan, ZHAN Jing, XU Zi-wei. Morphology-control and template-free fabrication of bimetallic Cu-Ni alloy rods for ethanol electro-oxidation in alkaline media [J]. Journal of Alloys and Compounds, 2021, 855: 157438.
[33] ISMAIL M I M. Green synthesis and characterizations of copper nanoparticles [J]. Materials Chemistry and Physics, 2020, 240: 122283.
[34] NIKOLIC N D, AVRAMOVIC L, IVANOVIC E R, MAKSIMOVIC V M, BASCAREVIC Z, IGNJATOVIC N. Comparative morphological and crystallographic analysis of copper powders obtained under different electrolysis conditions [J]. Transactions of Nonferrous Metals Society of China, 2019, 29: 1275-1284.
[35] LI Qi, GUO Jiang-nan, ZAHO Jun, WANG Can-can, YAN Feng. Porous nitrogen-doped carbon nanofibers assembled with nickel nanoparticles for lithium-sulfur batteries [J]. Nanoscale, 2019, 11: 647-655.
苗泽林1,湛 菁1,2,徐子伟1
1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 难冶有色金属资源高效利用国家工程实验室,长沙 410083
摘 要:针对反应体系Cu2+-Ni2+-NH3-NH4+-C2O42--H2O中Cu2+和Ni2+的反应行为,根据物质守恒原理,建立热力学数学模型。模拟计算结果表明,该体系中金属离子的沉淀是一个复杂的动态平衡过程;在高pH条件下,Cu2+和Ni2+与NH3配合分别形成[Cu(NH3)n]2+ (n=3-5)和 [Ni(NH3)m]2+ (m=3-6)的反应占主导地位。当[C2O42-]T=0.6 mol/L时,Cu2+和Ni2+的共沉淀pH范围在[NH3]T为0.6 mol/L和4.2 mol/L时分别为2.0-6.5和2.0-5.5。在pH>7.0的纯水体系中,由于Cu2+和Ni2+的分步沉淀,Cu-Ni草酸复盐热分解后得到的产物为Cu-Ni复合物。改变沉淀介质为混合溶剂(水/乙醇)可最终制备具有高纯度和良好结晶度的棒状Cu-Ni合金粉末。
关键词:配位-沉淀平衡;热力学数学模型;Cu-Ni 草酸复盐;Cu-Ni合金
(Edited by Xiang-qun LI)
Corresponding author: Jing ZHAN, Tel: +86-13975147556, E-mail: zhanjing@csu.edu.cn
DOI: 10.1016/S1003-6326(21)65591-7
1003-6326/ 2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press

