
Influence of sodium metanitrobenzene sulphonate on structures and
surface morphologies of phosphate coating on AZ91D
NIU Li-yuan(牛丽媛), LI Guang-yu(李光玉), JIANG Zhong-hao(江中浩),
SUN Li-ping(孙丽萍), HAN Dong(韩 冬), LIAN Jian-she(连建设)
Key Laboratory of Automobile Materials, Ministry of Education, College of Materials Science and Engineering,
Jilin University, Changchun 130025, China
Received 9 August 2005; accepted 23 March 2006
Abstract: The gray phosphate coating was formed on AZ91D magnesium alloy from the zinc phosphating bath containing sodium metanitrobenzene sulphonate in about 4 min. The structure, surface morphologies and phase compositions of the phosphate coatings were observed and analyzed by using SEM, XRD and EDS. It is shown that the phosphate coating becomes denser and has less micro holes with increasing the concentration of sodium metanitrobenzene sulphonate in the bath in the range of 2.0 to 6.0 g/L. The addition of sodium metanitrobenzene sulphonate greatly increases the micro cathode sites for the formation of the phosphate coating and decreases the porosity of the coating.
Key words: zinc phosphate coating; magnesium alloy; AZ91D; sodium metanitrobenzene sulphonate; phosphating process
1 Introduction
Recently many researches on light alloys have focused on magnesium alloy such as AZ31[1-3], AZ91[4-8] and AM60[9,10]. Many surface treatment coatings, including chemical conversion coating [3, 5, 11-13], anodizing[2,14,15], plating[16-19] and PVD coating[1], have been employed to improve the surface performances of magnesium alloys[20-22]. Among them, the technology of chemical conversion coatings is economical and popular in industry.
Chromate conversion coatings have been used to prevent the Mg alloy from corrosion and provide a base for the further paint. However, the hexavalent chromium used in the treatments baths is carcinogenic[13]. In consideration of the environment friendly technology, zinc phosphate coating should be one promising method for replacing the chromate conversion treatment.
Zinc phosphate coatings have been successfully used to underneath the paint of steel and aluminum for many years because they can enhance the adhesion of the paint and substrates[23-25]. However, it is difficult to obtain phosphate coating on magnesium alloys because of the electrochemical activity of magnesium. Although a lot of investigations focus on magnesium alloys, there are only limited studies about phosphate treatments on the surface of magnesium alloy. Phosphate coatings of Mg3(PO4)2 were obtained in phosphate-permanganate baths[11-13]. HAN et al[3] obtained a phosphate coating of Mn3(PO4)2 on AZ3lD magnesium alloy in a bath containing phosphate and manganese. Kouisni et al[9,10] studied the growth and the electrochemical behavior of zinc phosphate coatings containing mainly hopeite crystals on magnesium alloy AM60.
In the previous investigations[4,9,10], nitrite was used as the accelerating agents added in the phosphating bath. However, nitrite is also a carcinogen that should be forbidden to use. Therefore, the aim of this study is to use sodium metanitrobenzene sulphonate, SMBS(the abridge will be used in the follows)as the accelerating reagent to replace nitrite and its influence on the characteristics of the zinc phosphate coatings on AZ91D magnesium alloy was investigated. SEM and XRD were used to investigate the microstructures and compositions of the phosphate coatings. Zinc phosphating process and mechanism and coating thickness, porosity and anti- corrosion were investigated as well.
2 Experimental
AZ91D die-cast magnesium alloy samples with the size of 50 mm×50 mm×3 mm were used as substrate materials. The compositions of the magnesium alloy are given in Table 1.
Table 1 Compositions of AZ9lD magnesium alloy (mass fraction, %)

The samples were degreased in 10% KOH at 60 ℃and rinsed in de-ionized water to remove all alkali before the zinc phosphating treatment. The cleaned samples were then treated in the phosphating bath and dried. The phosphate coatings were obtained from the zinc phosphating bath (Table 2) at temperature of 40-45 ℃ and the pH value of 2.2 to 2.4. The pH of the phosphating bath was adjusted by NH3×H2O.
Table 2 Compositions of phosphating bath
The microstructure of the coating was observed using SEM (JSM-53l0, Japan Electronics) and EDS (INC250). The phase structures in phosphate coatings were analyzed by using XRD (D/max-2500PC, Cu Kα).
The mass per unit area of phosphate coating was measured by the following method. The phosphatized samples were dried and weighed. The phosphate coatings were eliminated in an alkaline bath (100 g/L sodium hydroxide) at 65-70 ℃ for about l5 min. As soon as the coatings were eliminated completely, the samples were dried and weighed again. The mass per unit area of the phosphate coating was calculated according to the equation: M=(M1-M2)/S, where M is the mass of the phosphate coating, M1 is the mass of phosphated magnesium alloy sample, M2 is the mass of sample after the phosphate coating being eliminated and S is the surface area of the magnesium alloy sample.
A porosity test of the phosphate coating was performed as follows. The samples of the die-cast AZ91D magnesium alloy with the size of 100 mm×100 mm×5 mm were phosphatized. A filter paper was soaked in a reagent solution of 10 g/L NaCl, 106 g/L ethanol and 0.1 g/L phenolphthalein dissolved in distilled water. The filter paper with the reagent solution was pasted onto the phosphate coating of magnesium alloy samples for 10 min. After taking the filter paper away, some red spots on the surface of the coating could be found. The area fraction of these red spots was considered to reflect the porosity of the phosphate coating.
3 Results and discussion
Phosphating reaction is a chemical reaction that occurs at the interface of metal and solution. The phosphating reaction stops once the surface of the metal is covered by the phosphate coating. The onset of phosphating reaction is thought to start with the dissolution of the metal ions into the solution. The stop of the phosphating reaction can be confirmed by the phenomenon that no hydrogen gas bubbles are observed to be given off on the metal surface.
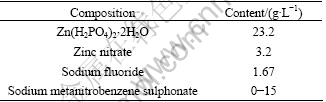
Fig.1 shows the influences of sodium metanitro- benzene sulphonate(SMBS) on the formation time of phosphate coating and the mass per unit area of phosphate coating. It is seen that the formation time of phosphate coating is about 55 min in the phosphating bath without SMBS. The formation time of phosphate coating is greatly decreased to 4-5 min when more than 4 g/L of SMBS is added in the phosphating bath. The accelerating mechanism of SMBS will be discussed latterly. The addition of SMBS also results in a decrease of the mass per unit area of phosphate coating from 7.5 g/m2 (without SMBS) to 4.5-5.0 g/m2 when more than 2.0 g/L of SMBS is added.
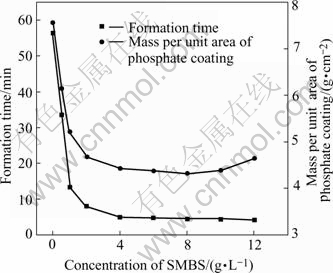
Fig.1 Variations of formation time of phosphate coating and mass per unit area of phosphate coating with concentration of sodium metanitrobenzene sulphonate in bath
Fig.2 shows the surface morphologies of phosphate coatings obtained from the phosphating baths containing 0, 2.0, 4.0 and 8.0 g/L SMBS. It can be seen that the phosphate crystals consist of some clusters and many small bright particles. EDS analysis on different micro regions indicates that the clusters are mainly hopeite (Zn3(PO4)2×4H2O) and the small bright particles are metallic zinc particles. It can be also observed that there are many micro cracks on the coating from the bath without SMBS (Fig.2(a)). The phosphate coating from the bath containing 2.0-4.0 g/L SMBS is crack-free and finer (Fig.2(b) and Fig.2(c)). However, some gaps or microcracks are observed along the boundaries of clusters on the phosphate coating obtained from the phosphating bath containing 8.0 g/L SMBS (Fig.2(d)). It can also be seen that the number of small bright particles decreases with the increasing of the SMBS content. There are least small bright particles on the phosphate coating from the bath containing 8.0 g/L SMBS (Fig.2(d)).
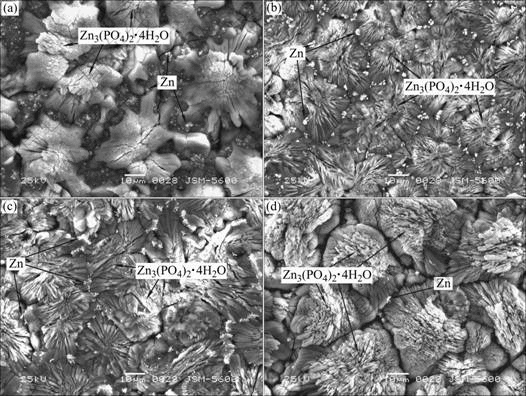
Fig.2 SEM morphologies of zinc phosphate coatings on AZ91D magnesium alloy obtained from phosphating baths with different concentrations of sodium metanitrobenzene sulphonate: (a) 0 g?L-1; (b) 2.0 g?L-1; (c) 4.0 g?L-1; (d) 8.0 g?L-1
The phase compositions in the coatings obtained from the phosphating baths with different concentration of SMBS were analyzed by XRD and the result is shown in Fig.3.
It is seen that the phases of phosphate coating consist of Zn3(PO4)2×4H2O (hopeite) and some metallic Zn crystals. Without the addition of SMBS, the random growth of zinc phosphate is observed. With the increase of SMBS concentration in the bath, the oriented growth of phosphate along (002) direction is gradually observed. The intensity of diffraction peaks of zinc crystal decreases as the concentration of SMBS in the phosphating bath increases, which indicates the decrease of content of metallic zinc in the phosphate coating.

Fig.3 XRD patterns of zinc phosphate coatings formed in phosphating baths containing different contents of sodium metanitrobenzene sulphonate: (a) 0 g?L-1; (b) 2.0 g?L-1; (c) 4.0 g?L-1; (d) 8.0 g?L-1
During phosphatization, the surface of sample is divided into micro anode sites (lower electron density sites) and micro cathode sites (higher electron density sites). The reactions on the surfaces should be thought to take place on different local polarization sites correspondingly. As to the AZ91D magnesium alloy, the finer β (Mg17Al12) phases distribute uniformly on the magnesium matrix. The b phase in the magnesium alloys is regarded as the micro cathode sites[9] and magnesium the micro anode sites.
In the case of without addition of SMBS in the phosphating bath, phosphate crystals are mainly formed on the micro cathode sites. In the bath both Zn(H2PO4)2×2H2O and Zn(NO3)2 are added, Zn(H2PO4)2× 2H2O is decomposed to
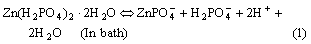
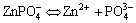 (In bath) (2)
(In bath) (2)
In the bath, Zn(NO3)2 also releases Zn2+:
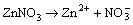 (In bath) (3)
(In bath) (3)
Zn2+ takes part in the coating formation reaction of Eqn.(5) or deposited on the anode sites (Eqn.(8)).
2H++2e→H2 (4)
The reduction of hydrogen results in the increase of local pH at metal-solution interface[4]. Where Zn2+ and 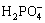 are the coating forming ions, which reacts on the surface of micro cathode sites to form phosphate coating:
are the coating forming ions, which reacts on the surface of micro cathode sites to form phosphate coating:
3Zn+2+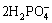 +4H2O→Zn3(PO4)3?4H2O+2H2 (5)
+4H2O→Zn3(PO4)3?4H2O+2H2 (5)
Some accelerators such as nitrate could consume hydrogen ions and make pH of the bath near the magnesium alloy surface rise rapidly and it is beneficial to the further formation of phosphate coating[4, 9].
The test results show that there should be 0.719 g/L Mg ions and 0.026 g/L Al ions in the phosphating bath after 1 m2 AZ91D alloy sample is phosphatized. There- fore, magnesium atoms and aluminum atoms dissolve and release metal ions at the micro anode sites:
Mg→Mg2++2e (6)
Al→Al2++2e (7)
Mg and Al dissolve in the bath to give out electrons, which reduces some Zn2+ in the bath to metallic Zn crystals on the micro anode sites and becomes the composition of the phosphate coating:
Zn2++2e→Zn (8)
When the magnesium alloy surface is fully covered by zinc phosphates and zinc, the phosphatization reactions stop.
SMBS is often used in the electroplating of metal as surfactant. Because SMBS is an anion surfactant, when
added in the phosphate bath the molecules of SMBS will be absorbed on the micro anode sites to act as micro cathode sites. Therefore, SMBS in the phosphating bath increases the amount of micro cathode sites. As a result, the fine and dense zinc phosphate (hopeite) crystals form on the magnesium alloy substrate. The phosphatization process is then accelerated (see Fig.1). On the other hand, the absorption of SMBS on the micro anode sites restrains the dissolution of magnesium and the deposition of zinc. The mass per unit area of phosphate coating obtained from the bath containing 1.0-8.0 g/L SMBS decreases with the increase of SMBS content because phosphate crystals become denser (see Fig.1). Consequentially, the crystal zinc content in the phosphate coating decreases with the addition of SMBS in the bath (as shown in Fig.3). SMBS accelerates the reaction because it absorbs on the anode surface and increases the ratio of area of cathode. It restrains reaction (8) because it absorbs on the micro anode sites and causes less Mg and Al dissolving into the bath.
Fig.4 shows the results of the porosity test on the phosphate coatings obtained from the phosphating bath with different concentration of SMBS. The porosity of the coating decreases greatly when SMBS is added in the bath. However, when more SMBS (for example, 8.0 g/L) is added, the porosity of the coating increases slightly. The test results of porosity are in agreement with the above microscopic observation of the morphologies of the coatings (Fig.2). Also, excessive SMBS in the bath produces sludge in the bath, which debases the surface quality of the coating. Therefore, the acceptable sodium metanitrobenzene sulphonate content in the phosphate bath is 2.0-6.0 g/L.
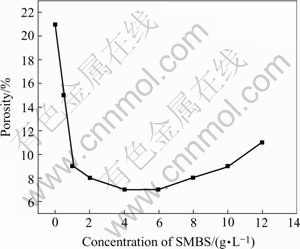
Fig.4 Variation of porosity (denoted by area fraction of red corrosion spots) with concentration of sodium metanitro- benzene sulphonate (SMBS) in bath
4 Conclusions
1) Phosphate coating is formed on AZ91D magnesium alloy in the present phosphating bath. The optimal bath compositions are 23.2 g/L Zn(H2PO4)2× 2H2O, 3.2 g/L zinc nitrate, 1.7 g/L sodium fluoride and 2.0-6.0 g/L sodium metanitrobenzene sulphonate in order to obtain the high quality zinc phosphate coating in 4-5 min. The zinc phosphate (hopeite) is the main phase of the coating and there are some crystal particles of zinc in the coating. With the increase of SMBS concentration in the bath, the Zn3(PO4)2×4H2O grows orientally along (002) direction and the content of metallic zinc rapidly decreases.
2) An anion surfactant, sodium metanitrobenzene sulphonate, is added in the phosphating bath to accelerate the phosphating reaction. The results show that the addition of sodium metanitrobenzene sulphonate greatly shortens the formation time of phosphate coating from 55 min to 4-5 min. The phosphate coating becomes denser and has less micro holes with increasing the concentration of sodium metanitrobenzene sulphonate in the bath in the range of 2.0-6.0 g/L.
3) The phosphating process can be accelerated because sodium metanitrobenzene sulphonate is absorbed on the micro anodic areas of the magnesium alloy to restrain the resolving of the substrate magnesium and to facilitate the nucleation of zinc phosphate. As a result, the dense and fine phosphate crystals form on the magnesium alloy substrate.
References
[1] Hollstein F, Wiedemann R, Scholz J. Characteristics of PVD-coatings on AZ31hp magnesium alloys [J]. Surf Coat Technol, 2003, 162(2-3): 261-268.
[2] Chiu L H, Chen C C, Yang C F. Improvement of corrosion properties in an aluminum-sprayed AZ31 magnesium alloy by a post-hot pressing and anodizing treatment [J]. Surf Coat Technol, 2005, 191(2–3): 181 – 187.
[3] Han E H, Zhou W Q, Shan D Y, KE W. Corrosion and Protection of magnesium alloy AZ31D by a new conversion coating [J]. Mater Sci Forum, 2003, 419(4): 879-883.
[4] Niu L Y, Jiang Z H, Li G Y, GU C D, LIAN J S. A study and application of zinc phosphate coating on AZ91D magnesium alloy [J]. Surf Coat Technol, 2006, 200: 3021-3026.
[5] Huo H W, Li Y, Wang F H. Corrosion of AZ91D magnesium alloy with a chemical conversion coating and electroless nickel layer [J]. Corros Sci, 2004, 46(6): 1467-1477.
[6] Ambat R, Aung N N, Zhou W. Evaluation of microstructural effects on corrosion behaviour of AZ91D magnesium alloy [J]. Corros Sci, 2000, 42(8): 1433-1455.
[7] HUANG Zheng-hua, GUO Xue-feng, ZHANG Zhong-ming. Effects of Ce on damping capacity of AZ91D magnesium alloy [J]. Trans Nonferrous Met Soc China, 2004, 14(2): 311-315.
[8] SONG Guang-ling, Atrens A, WU Xian-liang, ZHANG Bo. Corrosion behaviour of AZ21, AZ501 and AZ91 in sodium chloride [J]. Corros Sci, 1998, 40(10): 1769-1791.
[9] Kouisni L, Azzi M, Zertoubi M, DALARD F, MAXIMOVITCH S. Phosphate coatings on magnesium alloy AM60 part 1: study of the formation and the growth of zinc phosphate coatings [J]. Surf Coat Technol, 2004, 185(1): 58-67.
[10]Kouisni L, Azzi M, Dalard F, Maximovitch S. Phosphate coatings on magnesium alloy AM60 Part 2: Electrochemical behaviour in borate buffer solution [J]. Surf Coat Technol, 2005, 192(2-3): 239-246.
[11]Hawke D, Albright D L. A phosphate-permanganate conversion coating for magnesium [J]. Met Finish, 1995, 93(10): 34-38.
[12]Umehara H, Takaya M, Terauchi S. Chrome-free surface treatments for magnesium alloy [J]. Surf Coat Technol, 2003, 169-170: 666-669.
[13]Kwo Z C, Teng S S. Conversion-coating treatment for magnesium alloys by a permanganate-phosphate solution [J]. Mater Chem Phys, 2003, 80(1): 191-200.
[14]WANG Li-shi, CAI Qi-zhou, WEI Bo-kang, LIU Quan-xin. Charac- terization of oxide coatings formed on magnesium alloys using bipolar pulse microarc oxidation in phosphate solutions [J]. Trans Nonferrous Met Soc China, 2005, 15(3): 600-605.
[15]WANG Li-shi, CAI Qi-zhou, WEI Bo-kang, LIU Quan-xin. Electro- chemical performance of microarc oxidation coatings formed on AZ91D alloy in two group electrolytes [J]. Trans Nonferrous Met Soc China, 2005, 15(4): 777-783.
[16]Delaunois F, Petitjean J P, Lienard P, JACOB-DULIERE M. Autocatalytic electroless nickel-boron plating on light alloys [J]. Surf Coat Technol, 2000, 124(2-3): 201-209.
[17]Yerokhin A L, Shatrov A, Samsonov V, SHASHKOV P, LEYLAND A, MATTHEWS A. Fatigue properties of keronite coatings on a magnesium alloy [J]. Surf Coat Technol, 2004, 182(1): 78-84.
[18]LI Jian-zhong, SHAO Zhong-cai, TIAN Yan-wen. Electroless nickel plating on magnesium alloy in solution with NiSO4 as main salt [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(1): 152-156.(in Chinese)
[19]HU Bo-nian, CHEN Jue-ling, YU Gang, LIU Zheng, YE Li-yuan. Corrosion behavior of magnesium alloy in eletroless nickel plating bath [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(3): 463-470. (in Chinese)
[20]Funatania K. Emerging technology in surface modification of light metals [J]. Surf Coat Technol, 2000, 133-134: 264-272.
[21]Gray J E, Luan B. Protective coatings on magnesium and its alloys—A critical review [J]. J Alloy Compd, 2002, 336(1-2): 88-113.
[22]YU Gang, LIU Yue-long, LI Ying, YE Li-yuan, GUO Xiao-hua, ZHAO Liang. Corrosion and protection of magnesium alloys [J]. The Chinese Journal of Nonferrous Metals, 2002, 12(6): 1087-1097.(in Chinese)
[23]li Guang-yu, NIU Li-yuan, LIAN Jian-she, JIANG Zhong-hao. A black phosphate coating for C1008 steel [J]. Surf Coat Technol, 2004, 176(2): 215-221.
[24]Lazzarotto L, Maréchal C, Dubar L, DUBOIS A, OUDIN J. The effects of processing bath parameters on the quality and performance of zinc phosphate stearate coatings [J]. Surf Coat Technol, 1999, 122(2-3): 94-100.
[25]Akhtar A S, Susac D, Glaze P, WONG K C, WONG P C, MITCHELL K A R. The effect of Ni2+ on zinc phosphating of 2024-T3 Al alloy [J]. Surf Coat Technol, 2004, 187(2-3): 208-215.
Foundation item: Project(2004CB619301) supported by the National Basic Research and Development Program and Project 985-Automotive Engineering of Jilin University
Corresponding author: LIAN Jian-she; Tel: +86-431-5095875; E-mail: lianjs@jlu.edu.cn
(Edited by YUAN Sai-qian)