
Grain refinement of Mg-Al magnesium alloys by carbon inoculation
WANG Zhao-hui(王朝辉), KANG Yong-lin(康永林), ZHAO Hong-jin(赵鸿金), XU Yue(徐 跃)
School of Materials Science and Engineering, University of Science and Technology Beijing,
Beijing 100083, China
Received 28 July 2006; accepted 15 September 2006
Abstract: C2Cl6 was used as grain refiner for AM60 magnesium alloys. The effects of grain refinement process on chemical composition, microstructure, impact energy, hardness and mechanical properties of magnesium alloys were investigated with XRF spectrometer, optical and electronic microscopes, pendulum impact tester, hardness tester and MTS material testing machine. The results show that C2Cl6 has good effects on microstructure and mechanical properties of AM60 magnesium alloys. The optimum usage of C2Cl6 in AM60 for getting the best properties is 1.0%. The results of electronic microscopic examination and theoretical analyses show that Al4C3 should be the potent heterogeneous nucleant for Mg-Al magnesium alloys.
Key words: Mg-Al alloys; grain refinement; carbon inoculation; mechanical properties; mechanism
1 Introduction
Magnesium alloys have been widely used in recent years because of its low density, high specific strength and stiffness, attractive mechanical properties, good electromagnetic shielding, good thermal conductivity, excellent machine ability and availability. These properties fulfill the demands for low specific mass materials for prime consumer electronics and automotive parts. In Europe and North America, the USCAR and other programs have been carried out, and magnesium alloys have been used in motor vehicles as drive train parts, interior parts and body parts[1-2]. And most commercial magnesium alloys are based on the magnesium-aluminum system and casting is currently the most commonly used production process for magnesium components. They have good castability and exhibit good mechanical properties[3].
Grain refinement is the important practice for refining microstructure and elevating the mechanical properties of magnesium alloys. It is an essential and fundamental approach, since grain size significantly influences the mechanical properties of the castings, and the grain size is usually determined at an early stage of solidification by nucleation of the dendrites.
For magnesium-aluminum magnesium alloys, the carbon inoculation process is a practical method as one of the grain refinement processes. Carbon powder, Al4C3, C2Cl6 and other compounds containing carbon were used for refining the grain and improving mechanical properties of magnesium-aluminum alloys by some scholars. And the mechanism for grain refinement of Mg-Al magnesium alloys by carbon and other compounds containing carbon have been investigated. The Al4C3 or Al-C-O hypotheses are still the reasonable mechanisms for the grain refinement of Mg-Al magnesium alloys by carbon and other compounds containing carbon [4-9].
C2Cl6 was used as grain refiner for AM60 magnesium alloys in this work. And the grain refinement mechanism of C2Cl6 was discussed.
2 Experimental
AM60 magnesium alloys were used in the experiments. The grain refiner is C2Cl6 (analytically pure). The range of C2Cl6 addition is from 0.8% to 1.5% of the molten magnesium alloys. The stainless steel crucible and electrical resistance furnace were used for melting magnesium alloys.
The clean magnesium ingots about 1kg was put into the preheated crucible and heated to about 740 ℃. Then the C2Cl6 was added into the molten magnesium alloys with immersion bell. The molten magnesium alloys were refined with refinement flux after the C2Cl6 reacted with magnesium alloys completely. After held for about 20 min at this temperature, the molten magnesium alloys were cooled down to about 650 ℃ and poured into a permanent mold preheated to 200 ℃.
During the whole experimental procedures the crucible was covered with lid and the argon and flux were used for preventing the oxidation and burning of the magnesium alloys.
The chemical compositions of the alloys were obtained by using a Philips PW2400 XRF spectrometer. Samples for optical microscopy experiments were prepared following standard procedures. Specimens were first mechanically polished and finally etched in acetic picral and observed under a Laitz-DMRX microscope. In order to observe the nucleant of alloys, another samples were treated by solution heat treatment with the temperature of 410 ℃ for 12 h. Then the samples were polished and observed by LEO 1530 scanning electronic microscope. The hardness of samples was obtained by using HB-3000B Brinell hardness tester with a load of 2 452 N and a holding time of 30 s. In accordance with the GB/T228-2002 national standard for tensile tests, specimens were prepared with a diameter of 6 mm and a gage length of 30 mm. Tensile tests were performed on a MTS 810 testing machine at room temperature. The transverse section of sample for impact test is 10 mm×10 mm. And the impact tests were done with pendulum impact tester at room temperature.
3 Results and discussion
3.1 Chemical composition
The chemical composition of AM60 alloys under different melt conditions are shown in Table 1. It can be concluded that the Al content of AM60 magnesium alloys only decrease by 0.07% when the addition of C2Cl6 is 1.0%. But the Al content of alloys decreases rapidly when the addition of C2Cl6 is over 1.2%. So the addition of C2Cl6 must be controlled to keep the Al content of AM60 alloys. The contents of Zn, Mn and Si elements didn’t change nearly with the C2Cl6 addition. And it can be found that the Cl content doesn’t increase with the C2Cl6 addition.
3.2 Microstructure and hardness
The microstructure of AM60 magnesium alloys under different melting conditions are shown in Fig.1. The influence of C2Cl6 addition on grain size and hardness are shown in Fig.2. We can find that the original grain size of the magnesium alloys is about 250 μm in C2Cl6 free AM60 alloys and the hardness is only 48.3. When the addition of C2Cl6 increases to 1.0%, the grain size of magnesium alloys reduces to about 70 μm and the hardness reaches up to 53.1. But the grain size increases to about 100 μm and the hardness decrease to about 49.5 when the addition of C2Cl6 increases to 1.5%.
3.3 Mechanical properties
The mechanical properties of AM60 magnesium alloys under different melting conditions are shown in Fig.3.σUTS (ultimate tensile strength) of AM60 alloys without grain refinement process is 214 MPa with δ (elongation) of 11.4%. Both σUTS and δ increased when the C2Cl6 is used. When the addition of C2Cl6 is 1.0%, σUTS of AM60 reaches up to 241 MPa, which is 27 MPa higher than that of C2Cl6 free alloys, and δ is 20.1%, which is 8.7% higher than that of C2Cl6 free alloys. But when the addition of C2Cl6 increases continuously, the mechanical properties begin to decrease, σUTS and δ decrease to 227 MPa and 15.5% when the addition of C2Cl6 increases to 1.5%.
The impact energy (Ak) of AM60 alloys under different melting conditions are shown in Fig.4. The impact energy of AM60 alloys without grain refinement process is 30.4 J. When the usage of C2Cl6 is 1.0%, the relative impact energy can reach up to 46.1 J, which is 15.7 J higher than that of C2Cl6 free AM60 alloys.
Table 1 Chemical composition of AM60 alloys under different melt conditions(mass fraction, %)


Fig.1 Microstructures of AM60 magnesium alloys: (a) C2Cl6 free; (b) 1.0% C2Cl6 added
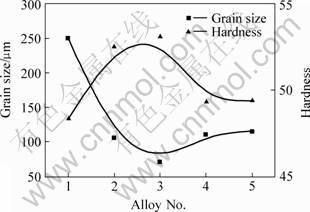
Fig.2 Dependences of grain size of AM60 and hardness on C2Cl6 addition
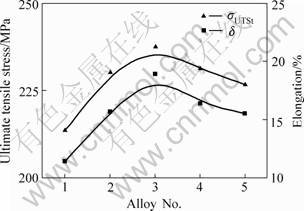
Fig.3 Dependences of σUTS and δ of AM60 on C2Cl6 addition
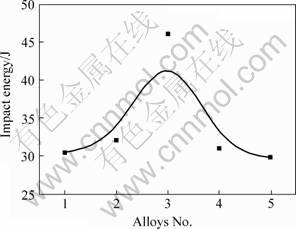
Fig.4 Dependence of impact energy of AM60 on C2Cl6 addition
3.4 Grain refinement mechanism
After C2Cl6 is added into molten Mg-Al magnesium alloys (at 740 ℃), the following reactions will occur:
C2Cl6== 2C+3Cl2 (1)
3C+4Al== Al4C3 (2)
The generated Al4C3 is regarded as the nucleant of α-Mg. But some scholars think that Al2OC will form in Mg-Al magnesium alloys, and it is also the nucleant of α-Mg[6]. The lattice parameters of α-Mg, Al4C3 and Al2OC are shown in Table 2.
Table 2 Lattice parameters of Mg, Al4C3 and Al2OC

Fig.5(a) show a SEM secondary electron image of a sample after solution heat treatment. The particle’s diameter is about 6 μm. Figs.5(b) and (c) are the EDS spectra of the particle and the matrix, respectively. Therefore, the particle mainly consists of Al, C and O.
According to the theory of solidification and theoretical crystallography analysis done by ZHANG et al[10], it can be concluded that Al4C3 and Al2OC are both suitable as the nucleant for magnesium alloys.
Al4C3+ Al2O3== 3Al2OC (3)
Suppose the Al2OC may be formed according to reaction (3). According to the thermodynamic calculation done by JIN et al[11], the formation of Al2OC in magnesium alloys containing less than 10%Al is impossible because the value of α(Al2OC) is much less than 1 in reaction (3). The Al2OC can’t be formed in the alloys.
According to the analysis beyond, Al2OC is impossible to form in AM60 alloys. Therefore, the particle are originally Al4C3 particle, and the oxygen is introduced during the sample preparation process before SEM observation[7,12].
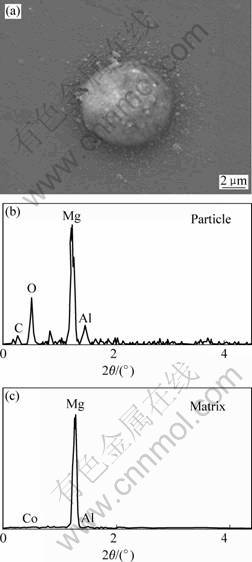
Fig.5 SEM image of particle (a) and EDS spectra (b), (c)
4 Conclusions
1) C2Cl6 was used as grain refiner for AM60 magnesium alloys. The results show that C2Cl6 has good effects on microstructure, hardness, impact energy and mechanical properties of AM60 magnesium alloys.
2) The optimum usage of C2Cl6 in AM60 for getting the best properties of AM60 magnesium alloys is 1.0%. Under this condition, the grain size can be reduced from 250 to 70 μm, and σUTS, δ and Ak can reach up to 241 MPa, 20.1% and 46.1 J, which are 27 MPa, 8.7% and 15.7 J higher than those of C2Cl6 free AM60 alloys, respectively.
3) The usage of C2Cl6 can influence the Al content in AM60 magnesium alloys. The Al content of alloys decreases rapidly when the addition of C2Cl6 is over 1.0%.
4) The electronic microscopic examinations and mechanism analysis show that Al4C3 should be the potent nucleant for α-Mg.
References
[1] SCHUMANN S. The paths and strategies for increased magnesium applications in vehicles[J]. Materials Science Forum, 2005, 488-489: 1-8.
[2] LI N. Magnesium advances and applications in North America automotive industry[J]. Materials Science Forum, 2005, 488-489: 931-935.
[3] DAHLE A K, LEE Y C, NAVE M D, SCHAFFER P L, STJOHN D H. Development of the as-cast microstructure in magnesium- aluminium alloys[J]. J Light Metals, 2001, 1: 61-72.
[4] EMLEY E F. Principles of Magnesium Technology[M]. Pergamon Press, 1966: 200-208.
[5] WALLACE J F, SCHWAM D, ZHU Y. The influence of potential grain refiner on magnesium foundry alloys[J]. AFS Transactions, 2003, 111: 1061-1075.
[6] YANO E, TAMURA Y, MOTEGI T, SATO E. Effect of carbon powder on grain refinement of a AZ91E magnesium alloy[J]. Materials Transactions, 2003, 44: 107-110.
[7] LU L, DAHLE A K, STJOHN D H. Grain refinement efficiency and mechanism of aluminium carbide in Mg-Al alloys[J]. Scripta Materialia, 2005, 53: 517-522.
[8] ZHANG S J, LI W X, YU K. A method of adding carbon containing flux for refining Mg alloy grain[J]. Special Casting & Nonferrous Alloys, 2002, 4: 18-19
[9] LIU Y H, LIU X F, LI T B, BIAN X F, ZHANG J Y. Grain refining effect of Al-Ti-C master alloy on Mg-Al alloys[J]. The Chinese Journal of Nonferrous Metals, 2003, 13(3): 622-625
[10] ZHANG M X, KELLY P M, QIAN M, TAYLOR J A. Crystallography of grain refinement in Mg-Al based alloys[J]. Acta Materialia, 2005, 53: 3261-3270.
[11] JIN Q L, EOM J P, LIM S G, PARK W W, YOU B S. Reply to comments on “Grain refining mechanism of a carbon addition method in a Mg-Al magnesium alloys”[J]. Scripta Materialia, 2005, 52: 421-423.
[12] CAO P, QIAN M, STJOHN D H. Native grain refinement of magnesium alloys[J]. Scripta Materialia, 2005, 53: 841-844
(Edited by CHEN Ai-hua)
Foundation item: Project(2002AA336080) supported by the Projects of Development Plan of the State High-technology Research of China
Corresponding author: WANG Zhao-hui; Tel: +86-10-62332335; E-mail: wwwzhaohui@sina.com