
ARTICLE
J. Cent. South Univ. (2019) 26: 1443-1448
DOI: https://doi.org/10.1007/s11771-019-4100-0
Synthesis and electrochemical properties of Li2FeSiO4/C/Ag composite as a cathode material for Li-ion battery
TANG Yi-qun(唐轶群)1, LIU Xi(刘喜)1, HUANG Xiao-bing(黄小兵)1, 2,
DING Xiang(丁祥)1, ZHOU Shi-biao(周诗彪)1, CHEN Yuan-dao(陈远道)1
1. Hunan Province Cooperative Innovation Center for the Construction &Development of Dongting
Lake Ecological Economic Zone, College of Chemistry and Materials Engineering,
Hunan University of Arts and Science, Changde 415000, China;
2. Key Laboratory of Preparation and Application of Environmental Friendly Materials of Ministry of Education, Jilin Normal University, Changchun 130103, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: Li2FeSiO4 is deemed to be a potential candidate for large-scale applications because of its abundance, low cost and high safety, etc. Unfortunately, its low conductivity, resulting in poor rate performance, has become a main obstacle to its applications in power battery and energy storage system. In this work, C-Ag coated Li2FeSiO4 is introduced to improve the innate electronic conductivity and Li-ion diffusion ability. The results demonstrate that Li2FeSiO4/C/Ag composite exhibits better electrochemical performance. It possesses a specific discharge capacity of 152, 121, 108 mA·h/g at 0.2C, 5C and 10C, respectively. At the same time, the Li2FeSiO4 /C/Ag composite shows good cycle stability and a capacity retention ratio of 97.9% after 100 cycles at 1C.
Key words: lithium-ion batteries; cathode material; Li2FeSiO4; pitch; C-Ag coating
Cite this article as: TANG Yi-qun, LIU Xi, HUANG Xiao-bing, DING Xiang, ZHOU Shi-biao, CHEN Yuan-dao. Synthesis and electrochemical properties of Li2FeSiO4/C/Ag composite as a cathode material for Li-ion battery [J]. Journal of Central South University, 2019, 26(6): 1443-1448. DOI: https://doi.org/10.1007/s11771-019-4100-0.
1 Introduction
After Thomas research group first reported orthogonal structure Li2FeSiO4 as cathode material for lithium ion battery in 2015, Li2FeSiO4 has been investigated with wide interests. It has significant advantages over traditional layered LiCoO2, spinel LiMn2O4 and olivine type LiFePO4 cathode materials [1-3]: 1) High theoretical specific capacity (332 mA·h/g) can be obtained if two lithium ions could be discharged reversibly; 2) It is clear that there are abundant, cheap and environmentally-friendly Si and Fe in the Earth's crust; 3) Si—O bonds are more stable and the security of Li2FeSiO4 is much higher. However, Li2FeSiO4 material is characterized by low electronic conductivity (10-14S/cm [4]) as well as low ionic diffusion coefficient (10-14 cm2/S [5]), which leads to undesirable properties of the battery at high rate, especially when this material applys to powervehicles, large energy storage batteries and other areas, it will be greatly restricted.
At present, four main methods were used by research groups to solve this problem, including: 1) a composite electrode is formed to heighten the electronic conductivity of the material by surface coating or dispersing the conductive carbon materials [6-8]; 2) ion doping of Li2FeSiO4, such as Sn4+ [9], P4+ [10, 11], Al3+ [12], Y3+ [13], Ti4+ [14], Mg2+ [5, 15], V5+ [16], Cr3+ [17] and Zn2+ [18], enhances intrinsic conductivity of materials; 3) the nanosized Li2FeSiO4 was synthesized [19-22] to shorten the Li ion migration path and increase the contact area of the electrode active material with the electrolyte; 4) the preparation of porous Li2FeSiO4 [23, 24] is beneficial to the electrolyte directly filling the pore, shortening the transmission distance of lithium ions, and reducing the damage of the material structure caused by volume expansion during cycling.
In our previous research, Li2FeSiO4/C composite was prepared from high softening point coal pitch as carbon source. Furthermore, our research finds that carbon obtained by carbonization of aromatic asphalt during heat treatment has higher graphitization degree and better electrical conductivity, which improves the electronic conductivity of Li2FeSiO4 [25]. Based on this research background, we adopted solid phase reaction to synthesize the Li2FeSiO4/C/Ag composite and studied the effects of C-Ag coating on the structure, morphology and electrochemical properties of Li2FeSiO4.
2 Experimental
Li2FeSiO4/C/Ag composite was synthesized from lithium carbonate (Aladdin, 99.99%), ferrous oxalate dihydrate (Shenzhen Shuotian Science and Technology, 99.7%), silver nitrate (Sinopharm Chemical Reagent Co., Ltd, 99.8%) and pitch (BTR Battery Materials Co., Ltd.). Firstly, 0.04 mol of lithium carbonate, 0.04 mol of ferric oxalate dihydrate, 0.04 mol of silica, 1.5 g of pitch and 0.14 g of silver nitrate were dispersed into 60 mL of acetone, adding 211 g zirconia beads of 3 mm diameter into zirconia ball mill tank with milling for 12 h at 500 rad/min. Secondly, the precursor was prepared by drying in a vacuum circumstance at 80 °C for 2 h. Finally, the precursor was preheated under argon atmosphere for 5 h at 350 °C, followed by heating at 700 °C for 10 h to acquire the Li2FeSiO4/C/Ag composite cathode material. The fabrication procedure of Li2FeSiO4/C composite is similar to that of Li2FeSiO4/C/Ag composite without using silver nitrate as the starting materials.
The structure of the prepared samples was characterized by X-ray diffraction instrument (XRD, DX2700). The morphology was observed by using a scanning electron microscope (SEM, HITACHI S340). The material conductivity was investigated by four-point probe (RTS-9). Specific surface area meter (BET, SSA4200) was carried out to measure material specific surface area.
The electrode mixture consisted of active substance (Li2FeSiO4), Super-P and binder LA-132, and the mass ratio of all three in the electrode is 80:10:10. The preparation of the electrode sheet followed the following steps. First, Super-P was evenly dispersed in the binder LA-132. Then adding the active substance, the slurry was obtained after uniform mixing. The slurry was evenly coated on the aluminum foil with a medical scraper and made into a circle with a diameter of about 1.2 cm. In the end, the electrodes were baked in a vacuum oven for 16 h before use. The coin cells were prepared in dry glove boxes filled with argon by using lithium plate as anode, Celgard 2400 as separator and 1 mol/L LiPF6/EC:DEC:DMC (V:V:V=1:1:1) as the electrolyte. Galvanostatic charge and discharge cycle tests were carried out on LAND equipment with a potential range from 1.5 to 4.8 V at 25 °C.
3 Results and discussion
Figure 1 shows the XRD profiles of Li2FeSiO4/C/Ag and Li2FeSiO4/C composites. As observed, the main peaks of Li2FeSiO4/C/Ag and Li2FeSiO4/C composites are consistent with those reported by NISHIMURA [26], which indicates that the addition of pitch and silver nitrate to the precursor does not affect the formation of Li2FeSiO4 phase. Compared with Li2FeSiO4/C composite, the diffraction peak strength of Li2FeSiO4/C/Ag composite at 2θ=38° and 2θ=44° was obviously enhanced, which is likely to be caused by the simple Ag in Li2FeSiO4/C/Ag composite. Test results of carbon sulfur analyzer show that the carbon contents of Li2FeSiO4/C/Ag and Li2FeSiO4/C composite materials were 12.1% and 12.9%, respectively. The Ag content of Li2FeSiO4/C/Ag composite is calcaculated to be about 1%.
Figure 2 shows the SEM images of Li2FeSiO4/C/Ag and Li2FeSiO4/C composites, where Figure 2(a) shows Li2FeSiO4/C composite and Figure 2(b) shows Li2FeSiO4/C/Ag composite. It can be seen from the SEM images that Li2FeSiO4/C/Ag and Li2FeSiO4/C composites particles are all below 100 nm, suggesting that the nanoparticle size of silica raw material and the milling process are beneficial to the formation of small particles. In addition, Li2FeSiO4/C/Ag and Li2FeSiO4/C composites present agglomerating. The test results of BET indicate that the specific surface areas of Li2FeSiO4/C/Ag and Li2FeSiO4/C composites were 94.5 and 86.3 m2/g, respectively. According to Ref. [27], Li2FeSiO4/C/Ag composite with larger specific surface area and smaller particle size has better performance in shortening the Li migration path and increasing the contact area of the electrode active material with the electrolyte. The electronic conductivities of Li2FeSiO4/C/Ag and Li2FeSiO4/C composites were measured by four-probe, which were 5.98×10-2 and 2.13×10-2 S/cm, respectively. The results demonstrate that Ag coating could obviously improve the electrical conductivity of Li2FeSiO4/C composite.
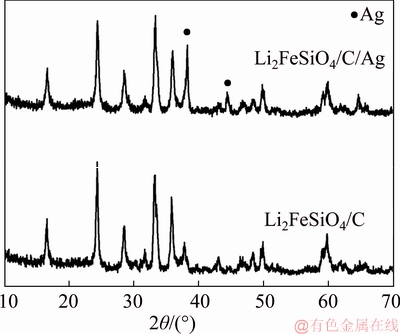
Figure 1 X-ray diffraction profiles of Li2FeSiO4/C/Ag and Li2FeSiO4/C composites
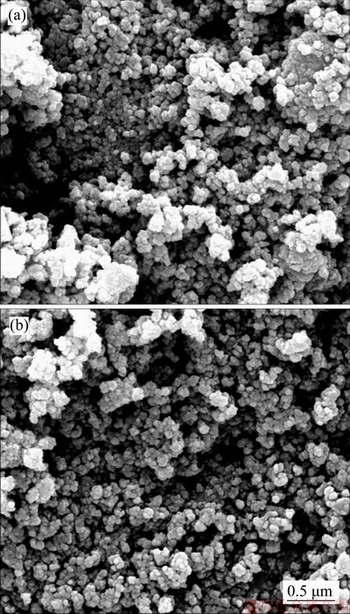
Figure 2 SEM images of Li2FeSiO4/C (a) and Li2FeSiO4/C/Ag composites (b)
Figure 3 depicts that the first profile of charge and discharge curves at various rates for Li2FeSiO4/C (a) and Li2FeSiO4/C/Ag (b) composites. As clearly seen, Li2FeSiO4/C/Ag and Li2FeSiO4/C composites have similar charge and discharge platforms corresponding to deintercalation lithium and intercalation lithium reactions. Meanwhile, the discharge voltage plateau decreases with the increase of current rate, demonstrating an increased polarization for both as-prepared Li2FeSiO4 samples [28]. In addition, the gap of Li2FeSiO4/C/Ag composite is narrower between charge voltage and discharge voltage at each rate than Li2FeSiO4/C composite, which indicates that Li2FeSiO4/C/Ag composite has less polarization [29].
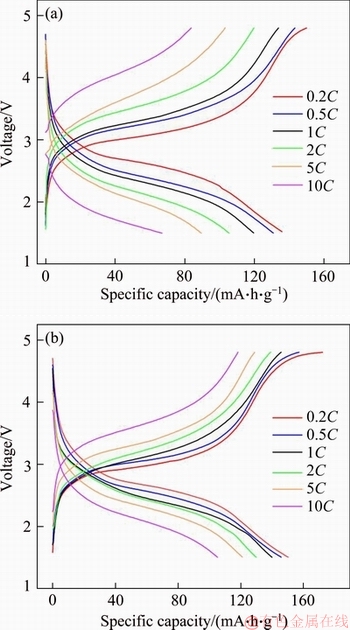
Figure 3 First profile of charge-discharge curves at various rates for Li2FeSiO4/C (a) and Li2FeSiO4/C/Ag (b)
Figure 4 compares the rate capability of Li2FeSiO4/C and Li2FeSiO4/C/Ag composites. As the charge and discharge current increase, the difference of discharge specific capacity between Li2FeSiO4/C composite and Li2FeSiO4/C/Ag composite gradually increases. For instance, at a low rate of 0.2C, Li2FeSiO4/C/Ag composite material delivers a discharge specific capacity of 152 mA·h/g, and Li2FeSiO4/C composite material shows a discharge capacity of 140 mA·h/g. At high rate, especially, at 5C and 10C, Li2FeSiO4/C/Ag composite possesses the discharge specific capacity of 121 and 108 mA·h/g, respectively. While Li2FeSiO4/C composite material has the discharge specific capacity of 89 and 68 mA·h/g, respectively. The results reveal that Li2FeSiO4/C/Ag composites have good rate performance. The reason for this phenomenon could be due to that the presence of Ag inhibits the sintering and the agglomeration of the Li2FeSiO4 particles, leading to a decrease in crystallinity of Li2FeSiO4, fining the material particles and increasing the specific surface area. Thereby, the Li transport path is shortened and the contact area of the electrode active material with the electrolyte is increased. At the same time, the simple Ag coating on the surface of Li2FeSiO4 improves its electronic conductivity.
The long cycle property at 1C for Li2FeSiO4/C/Ag composite is shown in Figure 5. From the image, the initial discharge specific capacity of Li2FeSiO4/C/Ag composite was 140 mA·h/g at 1C. After the 100th cycle, the discharge capacity of Li2FeSiO4/C/Ag composite remained at 97.9%, which indicates that Li2FeSiO4/C/Ag composite possesses excellent cyclic properties.
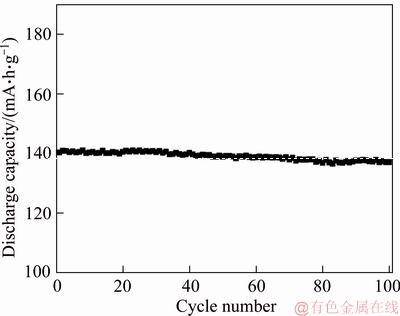
Figure 4 Rate performance of Li2FeSiO4/C/Ag and Li2FeSiO4/C composites
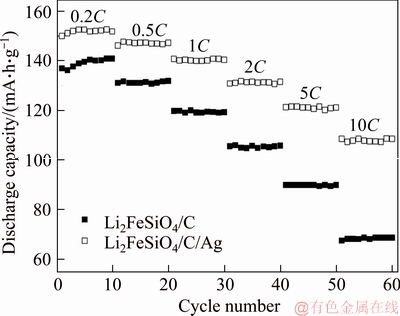
Figure 5 Long cycling performance at 1C for Li2FeSiO4/C/Ag composite
The Nyquist plots for Li2FeSiO4/C/Ag and Li2FeSiO4/C composites are displayed in Figure 6. The electrode testing was completed in 50% discharge state. There is a intermediate-frequency semicircle and a low-frequency straight line in the impedance spectrum. Among then, the intercepted resistance by the intersection of the high frequency region and the real axis represents the electrolyte resistance, corresponding to the ohm resistance (Re), and the high-frequency semicircle shows the charge transfer resistance (Rct). The oblique straight line at the low frequency region is in connection with the lithium ion diffusion coefficient [30-34]. By observing Figure 6, the charge transfer impedance of Li2FeSiO4/C/Ag and Li2FeSiO4/C composites electrodes was 75 and 132 Ω, respectively. At low rate, the charge transfer impedance has little impact on the discharge capacity of the material, but at high rate, the charge transfer impedance makes a considerable difference to the discharge capacity of the material. The Li2FeSiO4/C/Ag composite electrode has relatively smaller charge transfer impedance. Therefore, it has better rate performance. The results of AC impedance further confirm that Li2FeSiO4/C/Ag composite has good electrochemical property.
4 Conclusions
Li2FeSiO4/C/Ag composite has been synthesized by solid phase method. The results reveal that Li2FeSiO4/C/Ag composites have distinguished multiplying performance and cycle performance compared with Li2FeSiO4/C composite. At high current densities of 5C and 10C, the discharge capacities of the composite are 121 and 108 mA·h/g, respectively. After 100 cycles at 1C, the retention rate of the discharge ratio is 97.9%. The main reasons for Li2FeSiO4/C/Ag composite with superior electrochemical properties could be summarized as the presence of Ag can inhibit the sintering and the agglomeration of the Li2FeSiO4 particles, thus fining the material particles and making the specific surface area larger, further shortening the Li+ migration path and increasing the contact area of the electrode active material with the electrolyte. In addition, the electronic conductivity of material has a considerable increase by coating of Li2FeSiO4 material with simple Ag.
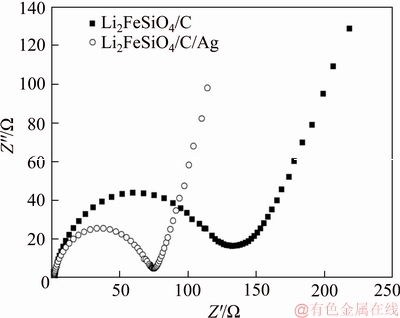
Figure 6 AC impedance plots of Li2FeSiO4/C/Ag and Li2FeSiO4/C composites
References
[1] LIU Xiao-yan, YANG Rong, DENG Kun-fa, REN Bing. Progress in the structure and modification of orthosilicates as cathode materials [J]. Battery Bimonthly, 2014, 44(5): 303-306. (in Chinese)
[2] BAO Li-ying, GAO Wei, SU Yue-feng, WANG Zhao, LI Ning, CHEN Shi, WU Fen. Progression of the silicate cathode materials used in lithium ion batteries [J]. Chinese Science Bulletin, 2013, 58(9): 783-792. (in Chinese)
[3] ZHU Hai, WU Xiao-zhen, LING Zan, ZHANG You-xiang. Superior electrochemical capability of Li2FeSiO4/C/G composite as cathode material for Li-ion batteries [J]. Electrochimica Acta, 2014, 117(4): 34-40.
[4] DOMINKO R. Li2FeSiO4 (M=Fe and/or Mn) cathode materials [J]. Journal of Power Sources, 2008, 184(2): 462-468.
[5] ZHANG S, DENG C, FU B L, YANG S Y, MA L. Doping effects of magnesium on the electrochemical performance of Li2FeSiO4 for lithium ion batteries [J]. Journal of Electroanalytical Chemistry,2010,644(2): 150-154.
[6] ZUO Peng-jian, WANG Tao, CHENG Guang-yu, CHENG Xin-qun, DU Chun-yu, YIN Ge-ping. Effects of carbon on the structure and electrochemical performance of Li2FeSiO4 cathode materials for lithium-ion batteries [J]. RSC Advances, 2012, 2(17): 6994-6998.
[7] WANG Kai, REN Wen-ju, YANG Jin-long, TAN Rui, LIU Yi-dong, PAN Feng. Depolarization effects of Li2FeSiO4 nanocrystals wrapped in different conductive carbon networks as cathodes for high performance lithium-ion batteries [J]. RSC Advances, 2016, 6(53): 47723-47729.
[8] ZHAO Yi, LI Jia-xin, WANG Ning, WU Chu-xin, DING Yun-hai, GUAN Lun- hui. In situ generation of Li2FeSiO4 coating on MWNT as a high rate cathode material for lithium ion batteries [J]. Journal of Materials Chemistry, 2012, 22(36): 18797-18800.
[9] WANG Kai, TENG Gao-feng, YANG Jin-long, TAN Rui, DUAN Yan-dong, ZHENG Jia-xin, PAN Feng. Sn(II,IV) steric and electronic structure effects enable self-selective doping on Fe/Si-sites of Li2FeSiO4 nanocrystals for high performance lithium ion batteries [J]. Journal of Materials Chemistry A, 2015, 3(48): 24437-24445.
[10] CHEN Wei-hua, ZHU Dan, LI Yan-yang, LI Chao-peng, FENG Xiang-ming, GUAN Xin-xin, YANG Chang-chun, ZHANG Jian-min, MI Li-wei. How to synthesize pure Li2-xFeSi1-xPxO4/C(x=0.03-0.15) easily from low-cost Fe3+ as cathode materials for Li-ion batteries [J]. Dalton Transactions, 2015, 44(33): 14805-14812.
[11] ARACHI Y, HIGUCHI Y, NAKAMURA R, TAKAGI Y, TABUCHI M. Synthesis and electrical property of Li2-xFeSi1-xPxO4 as positive electrodes by spark-plasma-sintering process [J]. Journal Power Sources, 2013, 244: 631-635.
[12] GAO Hai-yan, HU Zen, YANG Jin-gang, CHEN Jun. Li2-xFe1-xAlxSiO4/C nanocomposites cathodes for lithium-ion batteries [J]. Energy Technology, 2014, 2(4): 355-361.
[13] QIU Hai-long, YUE Hui-juan, JU Yan-ming, ZHANG Yong-quan, GUO Zhen-dong, WANG Chun-zhong, CHEN Gang, WEI Ying-jin, ZHANG Dong. Enhanced electrochemical performance of Li2FeSiO4/C positive electrodes for lithium-ion batteries via yttrium doping [J]. Electrochimica Acta, 2016, 188: 636-644.
[14] YANG Jin-long, ZHENG Jia-xin, KANG Xiao-chun, TENG Gao-feng, HU Lin, TAN Rui, WANG Kai, SONG Xiao-he, MU Shi-chun, PAN Feng. Tuning structural stability and lithium-storage properties by d-orbital hybridization substitution in full tetrahedron Li2FeSiO4 nanocrystal [J]. Nano Energy, 2016, 20: 117-125.
[15] QU L, LUO D, FANG S, QU Long, LUO Dong, FANG Shao-hua, LIU Yi, YANG Li, HIRANO Shin-ichi, YANG Chun-chen. Mg-dopedLi2FeSiO4/C as high-performance cathode material for lithium-ion battery [J]. Journal of Power Sources, 2016, 307: 69-76.
[16] HAO H, WANG J, LIU J, HAO Hao, WANG Jun-bo, LIU Jia-li, HUANG Tao, YU Ai-shui. Synthesis characterization and electrochemical performance of Li2FeSiO4/C cathode materials doped by vanadium at Fe/Si sites for lithium ion batteries [J]. Journal of Power Sources, 2012, 210(9): 397-401.
[17] ZHANG S, DENG C, FU B L, ZHANG S, DENG C, FU B L, YANG S Y, MA L. Effects of Cr doping on the electrochemical properties of Li2FeSiO4 cathode material for lithium-ion batteries [J]. Electrochimica Acta, 2010, 55(28): 8482-8489.
[18] DENG C, ZHANG S, YANG S Y, FU B L, MA L. Synthesis and characterization of Li2Fe0.97M0.03SiO4 (M=Zn2+, Cu2+, Ni2+) cathode materials for lithium ion batteries [J]. Journal of Power Sources, 2011, 196(1): 386-39.
[19] GONG Z L, LI Y X, HE G N, LI J, YANG Y. Nanostructured Li2FeSiO4 electrode material synthesized through hydrothermal-assisted sol-gel process [J]. Electrochemical Solid-State Letters, 2008, 11(5): A60-A63.
[20] ZHANG Sen, DENG Chao, YANG Sai-yu. Preparation of nano-Li2FeSiO4 as cathode material for lithium-ion batteries [J]. Electrochemical Solid-State Letters, 2009, 12(7): A136-A139.
[21] YAN Zi-peng, CAI Shu, ZHOU Xing, ZHAO Yong-ming, MIAO Li-juan. Sol-gel synthesis of nanostructured Li2FeSiO4/C as cathode material for lithium ion battery [J]. Journal of the Electrochemical Society, 2012, 159(6): A894-A898.
[22] RANGAPPA D, MURUKANAHALLY K D, TOMAI T, UNEMOTO A, HONMA I. Ultrathin nanosheets of Li2MSiO4 (M=Fe, Mn) as high-capacity Li-ion battery electrode [J]. Nano Letters, 2012, 12(3): 1146-1151.
[23] HASEGAWA G, SANNOBE M, ISHIHARA Y, KANAMORI K, NAKANISHI K, ABE T. New Li2FeSiO4–carbon monoliths with controlled macropores: Effects of pore properties on electrode performance [J]. Physical Chemistry Chemical Physics Pccp, 2013, 15: 8736-8743.
[24] CHEN Zhong-xue, QIU Shen, CAO Yu-liang, QIAN Jiang-feng, AI Xin-ping, XIE Kai, HONG Xiao-bin, YANG Han-xi. Hierarchical porous Li2FeSiO4/C composite with 2 Li storage capacity and long cycle stability for advanced Li-ion batteries [J]. Journal of Materials Chemistry A, 2013, 1(16): 4988-4992.
[25] HUANG Xiao-bing, LI Xing, WANG Hai-yan, PAN Zhong-lai, QU Mei-zhen, YU Zuo-long. Synthesis and electrochemical performance of Li2FeSiO4/C as cathode material for lithium batteries [J]. Solid State Ionics, 181(2010): 1451-1455.
[26] NISHIMURA S C, HAYASE S, KANNO R. Structure of Li2FeSiO4 [J]. J Am Chem Soc, 2008, 130, 13212-13213.
[27] AVCI E, MAZMAN M, UZUN D, BICER E, SENER T. High performance LiFePO4/CN cathode material promoted by polyaniline ascarbon–nitrogen precursor [J]. Journal of Power Sources, 2013, 240(1): 328-337.
[28] ZHANG Jia-wei, CAI Yu-rong, WU Jun, YAO Ju-ming. Graphene oxide-confined synthesis of Li4Ti5O12 microspheres as high-performance anodes for lithium ion batteries [J]. Electrochimica Acta, 2015, 165: 422-429.
[29] NIEN Y H, CAREY J R, CHEN J S. Physical and electrochemical properties of LiFePO4/C composite cathode prepared from various polymer-containing precursors [J]. Journal of Power Sources, 2009, 193: 822-827.
[30] SUN Dan, ZHU Xiao-bin, LUO Bin, ZHANG Yu, TANG You-gen, WANG Hai-yan, WANG Lian-zhou. New binder-free metal phosphide–carbon felt composite anodes for sodium-ion battery [J]. Advanced Energy Materials, 2018, 1801197.
[31] LV Yan-rong, ZHANG Lu, CHENG Gang, WANG Peng-fei, ZHANG Tian-ze, LI Chuan-chang, JIANG Ying-qiao, HE Zhang-xing, DAI Lei, WANG Ling. Preparation of carbon nanosheet by molten salt route and its application in catalyzing VO2+/VO2+ redox reaction [J]. Journal of the Electrochemical Society, 2019, 166 (6): A953-A959.
[32] XIAO Wei, WANG Zhi-yan, ZHANG Yan, FANG Rui, YUAN Zun, MIAO Chang, YAN Xue-min, JIANG Yu. Enhanced performance of P(VDF-HFP)-based composite polymer electrolytes doped with organic-inorganic hybrid particles PMMA-ZrO2for lithium ion batteries [J]. Journal of Power Sources, 2018, 382: 128-134.
[33] XIAO Wei, WANG Zhi-yan, MIAO Chang, MEI Ping, ZHANG Yan, YAN Xue-min, TIAN Ming-lei, JIANG Yu, LIU Jing-jing. Electrolytes doped with spherical-like and honeycomb structural Li0.1Ca0.9TiO3 particles [J]. Front Chem, 2018, 6: 525.
[34] HE Zhang-xing, LI Man-man, LI Yue-hua, LI Chuan-chang, YI Zao, ZHU Jing, DAI Lei, MENG Wei, ZHOU Hui-zhu, WANG Ling. ZrO2 nanoparticle embedded carbon nanofibers by electrospinning technique as advanced negative electrode materials for vanadium redox flow battery [J]. Electrochimica Acta, 2019, 309: 166-176.
(Edited by FANG Jing-hua)
中文导读
Li2FeSiO4/C/Ag复合正极材料的合成与性能研究
摘要:Li2FeSiO4正极材料因其资源丰富、成本低、安全性能高等优点备受研究者关注,但其电导率低,倍率性能较差,极大地限制在动力和储能领域的应用。针对上述科学问题,本文采用固相法合成C和Ag共包覆的Li2FeSiO4,以提高其电子电导率和离子扩散系数。结果表明:与Li2FeSiO4/C复合物相比,Li2FeSiO4/C/Ag复合物具有较好的电化学性能,在0.2C、5C和10C下的放电比容量分别为152、121和108 mA·h/g;1C下循环100次后,其放电容量保留为97.9%。
关键词:锂离子电池;正极材料;硅酸亚铁锂;沥青;碳-银共包覆
Foundation item: Project(21771062) supported by the National Natural Science Foundation of China; Project(2016JJ2092) supported by the Hunan Provincial Natural Science Foundation, China; Project(2019013) supported by the Open Project Program of Key Laboratory of Preparation and Application of Environmental Friendly Materials, Ministry of Education, Jilin Normal University, China
Received date: 2018-10-27; Accepted date: 2019-03-04
Corresponding author: HUANG Xiao-bing, PhD, Associate Professor; Tel: +86-13762681279; E-mail:hxb220170@126.com; ORCID: 0000-0003-2217-0601