
Development of microarc oxidation process to improve corrosion resistance on AZ91HP magnesium alloy
ZHANG Rong-fa(张荣发)1, 2, SHAN Da-yong(单大勇)2, HAN En-hou(韩恩厚)2, GUO Shi-bo(郭世柏)1
1. Jiangxi Key Laboratory of Surface Engineering, Jiangxi Science and Technology Normal University,Nanchang 330013, China
2. Environmental Corrosion Center, Institute of Metal Research, Chinese Academy of Sciences,Shenyang 110016, China
Received 10 April 2006; accepted 25 April 2006
Abstract: A new anodizing process, which does not contain chromate but can improve the corrosion resistance of magnesium alloys significantly, was developed using a microarc power supply. Surface morphology was observed and the coating was compact and ceramic-like. In addition, the corrosion resistance of samples before and after anodization by the new process and a method in US Patent 5470664 was compared by potentiodymaic polarization curves, electrochemical impedance spectroscopy (EIS) and salt spray test. The results show that the anodization can improve the corrosion resistance of magnesium alloy. The samples obtained by the new process and the method mentioned in the US Patent 5470664 achieve 9 and 7 rates after 336 h salt spray test, respectively.
Key words: magnesium alloy; corrosion resistance; microarc oxidation
1 Introduction
Among the practical metallic materials, magnesium and its alloys are the lightest and superior in the mechanical properties, so they offer various possibilities as regards applications in the automotive, electronic and aeronautical industries [1, 2]. However, since they are chemically active and inferior in corrosion resistance, their practical applications have been limited. Surface treatments are necessary to protect against corrosion. There are many surface modification technologies, applied to magnesium based substrates for improved corrosion and wear resistance, for example, electroless plating [3, 4], conversion coatings [5, 6], anodizing [7, 8], solid diffusion [9, 10] and laser surface alloying cladding [11]. It is generally accepted that the best corrosion resistance for magnesium and magnesium alloy surfaces is achieved by anodization [12]. In this paper, a new anodizing process to improve corrosion resistance of magnesium alloy considerably was developed. The corrosion resistance of magnesium alloy before and after anodization was tested by polarization curve, EIS and salt spray.
2 Experimental
The specimens were cut from AZ91HP ingot alloys, which chemical compositions were determined by inductively coupled plasma (ICP). The results are given in Table 1.
Table 1 Chemical compositions of AZ91HP magnesium alloy (mass fraction, %)

Samples for anodization were masked with sealant leaving 5 cm×6 cm. These samples were placed in a desiccator after they were polished successively on SiC paper up to 1 000 grit finish, degreased by acetone, washed with distilled water and dried in a cool air stream.
In order to compare with the new process, a method mentioned in US Patent 5470664, which results in a superior coating and has increased abrasion and corrosion resistance [13], was used in this paper. The used electrolytes were as follows: 8 g/L NaOH, 9 g/L KF?2H2O, 18 g/L Na2SiO3?9H2O. The new process contained phosphate ions, fluoride ions and borate ions.
The pretreated samples were anodized by homemade micro-arc oxidation equipment. The equipment consists of a MAOI-050 power supply, a stainless steel barrel and a stirring and cooling system that automatically controls the electrolyte temperature at (20±0.5)℃. The power supply can provide unipolar or bipolar current and the electric parameters, such as current density, frequency and duty cycle can be adjusted independently to obtain excellent anodic coatings. In our experiment, unipolar current was used under constant current mode. The same electric parameters, namely current density 20 mA/cm2, frequency 600 Hz, duty cycle 15%, were used for two processes. Final voltage of the method in Ref.[13] was 260 V and that of the new anodizing process was 440 V.
Morphologies of anodized samples were observed by XL30FEG ESEM after they were rinsed with distilled water and then dried in a cool air stream before spraying with gold. The compositions of anodic films were determined by EDX analysis attached to ESEM.
Coating thickness was measured by a 6000-FN1 eddy current instrument (Made in Hong Kong), which was calibrated with the same base metal. Measurements were carried out for 10 times and the coating thickness value is the average.
Polarization curves and EIS of the specimen were measured in 3.5%(mass fraction) NaCl solution. The counter electrode was platinum sheet and a saturated calomel electrode (SCE) was used as reference. The time of initial delay and the scan speed were 300 s and 2.5 mV/s, respectively. Scanning was started from -0.2 V versus open circuit potential (OCP) towards more noble direction until the breakdown of film occurred. The parameters used for measuring EIS were as follows: initial delay 1 800 s, the amplitude of AC signal 10 mV, the frequency 1.0×10-2-1.0×105 Hz.
In accordance with ASTM B117-95 and ASTM B537-70, salt spray test was conducted and corrosion resistance of anodic coatings was evaluated after 336 h.
3 Results and discussion
The surface morphologies of anodic coatings formed by the new process and the method in Ref.[13] are shown in Fig.1. Anodic coating obtained by the new process is compact and ceramic-like. A few circular pores of 2-8 mm diameter and 4-12 mm distance between them are present in the porous outer layer (Fig.1(a)).
For the anodic coating formed by the method in Ref.[13], coating pores are very uniform, generally 0.6-3.0 mm in diameter and 1.8-3.2 mm from each other (Fig.1(b)), which are all smaller than those by the new process.
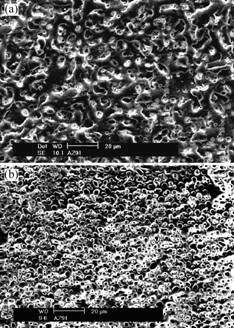
Fig.1 Surface morphologies of anodic coatings formed by new process (a) and method in Ref.[13] (b)
EDX analysis shows that the coating formed by the new process contains Mg, Al, O, F and P (Fig.2(a)) while the coating formed by the method in Ref.[13] contains Mg, Al, O, F and Si (Fig.2(b)), which come from both the electrolyte and the base metal.
The potentiodynamic polarization curves in 3.5% NaCl solution of bare and coated alloy obtained by the new process and the method in Ref.[13] are shown in Fig.3.
According to polarization curves, the corrosion current density decreased 2 orders for coating obtained by the new process and 1 order for coating obtained by the method in Ref.[13]. Although two anodizing processes can improve corrosion resistance of the substrate, the effect of the new process is more significantly than that of the method in Ref.[13].
EIS (as shown in Fig.4) clearly indicates that the diameter of high frequency capacitive loop for anodized sample by the new process is larger than that by the method in Ref.[13], which indicates that corrosion resistance of anodized sample obtained by the new process is better than that by the method in Ref.[13].
The results of salt spray tests are listed in Table 2. It can be seen that the results are consistent with the electrochemical results.
The pictures of anodized samples obtained by the two processes after 336 h salt spay test are shown in Fig.5.
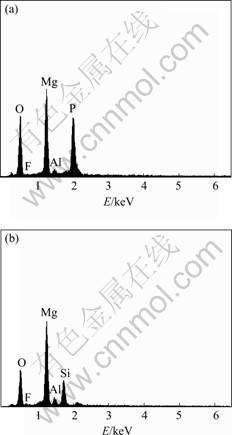
Fig.2 EDX spectra of AZ91HP anodized by new process (a) and method in Ref.[13] (b)

Fig.3 Potentiodynamic polarization curves of anodized samples and blank
After 336 h salt spray test, no obvious corrosion pits form on the anodized sample obtained by the new process (Fig.5(a)). However, many small pits appear on the sample surface obtained by the method in Ref.[13].
Three methods of evaluating corrosion resistance were in good agreement with each other, which indicated that anodization by the new process improved corrosion resistance of magnesium alloy considerably. The proper-
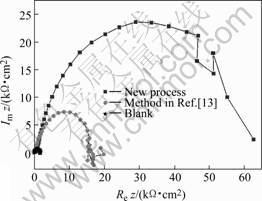
Fig.4 EIS of anodized samples and blank

Fig.5 Pictures of anodized samples by new process (a) and method in Ref.[13] (b) after 336 h salt spray test
ties of anodic coating on magnesium depend on many factors, such as the concentrations and compositions of electrolyte [14, 15], input electric parameters [16], and phase microstructure of substrate metal [17]. The electrolyte plays a main role in determining the coating properties, such as coating thickness, surface morphology, compositions and corrosion resistance. The reaction process of anodic coating during immersion in NaCl solution may include that the electrolyte penetrated into the porous layer with a high speed and then infiltrated into the inner barrier layer with a slow speed [18]. The main reason why the coating obtained by the new process is superior to the method in Ref.[13] may be that the coating by the former is thicker and compacter than that by the latter, which can suppress the
Table 2 Protection rating subjected to salt spray test for 336 h

transfer of corrosive electrolyte between the anodic coating and solution.
4 Conclusions
The new anodizing process can improve corrosion resistance of magnesium alloys considerably. After 336 h salt spray test, the anodized sample can achieve a rating of 9 and no obvious corrosion pits appear on its surface.
References
[1] FROES F H, ELIEZER D, AGHION E. The science, technology, and applications of magnesium [J]. JOM, 1998, 5(9): 30-34.
[2] DECKER R F. The renaissance in magnesium [J]. Advanced Materials and Processes, 1998, 9: 31-33.
[3] LI J z, TIAN Y w, HUANG Z q, ZHANG X. Studies of the porosity in electroless nickel depositis on magnesium alloy[J]. Applied Surface Science, 2006, 252: 2839-2646.
[4] GU C d, LIAN J s, HE J g, JIANG Z h, JIANG Q. High corrosion resistance nanocrystalline Ni coating on AZ91D magnesium [J]. Surface and Coatings Technology, 2006, 200: 5413-5418.
[5] LIN C S, LIN H C, LIN K M, LAI W C. Formation and properties of stannate conversion coatings on AZ61 magnesium alloys [J]. Corrosion Science, 2006, 48: 93-109.
[6] HAN E H, ZHOU W q, SHAN D y, KE W. Corrosion and protection of magnesium alloy AZ31D by a new conversion coating [J]. Materials Science Forum, 2003, 419-422: 879-882.
[7] GUO H F, AN M Z. Growth of ceramic coatings on AZ91D magnesium alloys by micro-arc oxidation in aluminate-fluoride solutions and evaluation of corrosion resistance [J]. Applied Surface Science, 2005, 246: 229-238.
[8] XIA S J, YUE R, RATEICK R G Jr, BIRSS V I. Electrochemical studies of AC/DC anodized Mg alloy in NaCl solution [J]. J Electrochem Soc, 2004, 151(3): B179-B187.
[9] MA Y p, XU K w, WEN W x, HE X p, LIU P f. The effect of solid diffusion surface alloying on properties of ZM5 magnesium alloy [J]. Surface and Coatings Technology, 2005, 190: 165-170.
[10] ZHU L q, SONG G l. Improved corrosion resistance of AZ91D magnesium alloy by an aluminium-alloyed coating[J]. Surface and Coatings Technology, 2006, 200: 2834-2840.
[11] LIU S Y, HU J D, YANG Y, GUO Z X, WANG H Y. Microstructure analysis of magnesium alloy melted by laser irradiation [J]. Applied Surface Science, 2005, 252: 1723-1731.
[12] OSTROVSKY, ILYA. Method of anodizing of magnesium and magnesium alloys and producing conductive layers on anodized surface [P]. US: 20030000847, 2003.
[13] BARTAK D E, LEMIEUX B E, WOOLSEY E R. Hard Anodic Coating for Magnesium Alloys [P]. US: 5470664, 1995.
[14] MA Y, NIE X, NORTHWOOD D O, HU H. Systemic study of the electrolytic plasma oxidation process on a Mg alloy for corrosion protection [J]. Thin Solid Films, 2006, 494: 296-301.
[15] FUKUDA H, MATSUMOTO Y. Effects of Na2SiO3 on anodization of Mg-Al-Zn alloy in 3 mol/L solution [J]. Corrosion Science, 2004, 46: 2135-2142.
[16] BLAWERT C, HEITMANN V, DIETZEL W, NYKYFORCHYN H M, KLAPKIV M D. Influence of process parameters on the corrosion properties of electrolytic conversion plasma coated magnesium alloys [J]. Surface and Coatings Technology, 2005, 200: 68-72.
[17] SHI Z m, SONG G l, ATRENS A. Influence of the β phase on the corrosion performance of anodized coatings on magnesium-aluminium alloys [J]. Corrosion Science, 2005, 47: 2760-2777.
[18] DUAN H p, DU K q, YAN C w, WANG F h. Electrochemical corrosion behavior of composite coatings of sealed MAO film on magnesium alloy AZ91D [J]. Electrochimica Acta, 2006, 51: 2898-2908.
(Edited by ZHAO Jun)
Corresponding author: ZHANG Rong-fa; Tel: +86-791-3801423; E-mail: rfzhang-10@hotmail.com