Layered structure of Ni-Al multi-layered metal-intermetallic composites fabricated by in-situ reactions
ZHANG Jiao(张 佼)1, 2, SUN Bao-de(孙宝德)1, XIA Zhen-hai(夏振海)3
(1. State Key Laboratory of Metal Matrix Composites, Shanghai Jiaotong University, Shanghai 200030, China;
2. School of Materials Science and Engineering, Hebei University of Technology, Tianjin 300132, China;
3. Division of Engineering, Brown University, Providence, USA)
Abstract: Systematical experiments were done at five temperature levels: 500℃, 630℃, 900℃, 1000℃ and 1100℃ to illuminate the layer structure of the multi-layered metal-intermetallic composites of Ni-Al system that were fabricated by a previously reported simple and cost-effective method. The analysis of back scattering photos and XRD examination of specimens reveal that the look like single compound layer is composed of several different components. The primary phase produced during reaction is Ni2Al3 and there exists a like two-phase field between NiAl3 and Ni2Al3. The high temperature phases like NiAl and Ni3Al are also found at low temperature. The results indicate that the key driving force of in-situ reaction is not temperature, but the atom concentration.
Key words: intermetallic compound; in-situ reaction; layered structure; back scattering photo CLC number: TG111.6
Document code: A
1 INTRODUCTION
Most intermetallic compounds have high strength, excellent oxidation resistance at high temperature[1-6]. However, the inherent low ductility and low fracture toughness at ambient temperature limit them to be used in engineering. Some of them, such as ferrite aluminides, nickel aluminides and titanium aluminides, were in depth studied for practical utilization. In order to improve their comprehensive mechanical properties, various methods had been attempted[7-9]. The recent research on laminated structure composites enlightened us to adopt a new method to improve the properties of nickel aluminides. ZHU et al[10] reported that the diffusion reactions between nickel and aluminum were triggered at high temperature, and the produced compound layers jointed the metal layers together. Similar work was reported in Ref.[11]. In our previous work[12], the laminated metal-intermetallic composites had been fabricated by interlayer in-situ reactions, and had been proved to have great improvement in properties over the constituent elements. Though the previous works[10-12] showed the distinct layered structures, the details of structure were not made clear. In this paper a systematical experiment was designed to reveal the details of the compound layers. The results indicate that the single compound layer under SEM is actually composed of several different compounds, and the number of compound layers is maximal when the reaction temperature is under the melting point of Ni2Al3. Further more, the high temperature phases are also found in low temperature samples.
2 EXPERIMENTAL
2.1 Preparation of samples
In the experiments, foils of commercially pure nickel(≥99.98%, mass fraction) and aluminum(≥99.93%, mass fraction) were used to stack alternative Ni and Al layers. The thickness of aluminum foils was 0.1mm and nickel foils 0.05mm. Ni and Al foils were cut to size of 10mm×80mm. About 50 foils of Ni and Al were stacked alternatively. The flow chart of the process is introduced in details in Fig.1. The pieces of nickel foil and aluminum foil were pre-cleaned in 5% alkaline solution first to remove oil and other organic contaminants, then in 15%-20% hydrochloric acid. After being rinsed by distilled water and dried at ambient temperature, the sheets were stacked alternately into Ni/Al multi-layer samples which were pressed with a pressure of 200MPa, and held in a graphite mould, so that the metal sheets could keep contact with each other closely.
The pressed multi-layer samples were then treated in a self-made vacuum furnace(as shown in Fig.2) with a vacuum of 5×10-3Pa.
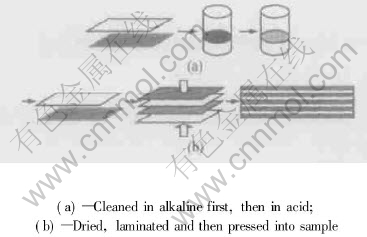
Fig.1 Flow chart of pretreatment
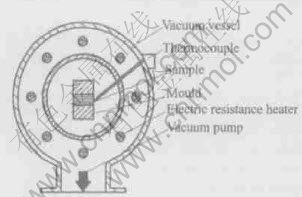
Fig.2 Sketch of equipment
2.2 Fabrication
In order to study the reaction status and results under different temperatures, the treating method was divided into five levels: 500℃, 630℃, 900℃, 1000℃ and 1100℃. The temperature was elevated and held strictly along the specified technological routes that were shown in Fig.3. The pre-treatment at 300℃ was a necessary procedure before fabrication[12]. When the experiments were finished, the samples cooled in furnace in vacuum environment. According to our previous results[12,13], the original aluminum layers had been used up after 2h treating at 630℃. Therefore, the aluminum would not melt or leak out when the temperature went up to 900℃, 1000℃ and 1100℃.
2.3 Microstructure determination
After fabrication, the specimens were cut and
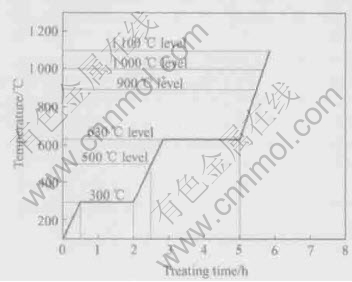
Fig.3 Technical routes of elevating temperature
polished. The details of layered microstructure were examined under PHILIP505 scanning electronic microscope(SEM) by back scattering observation. The phase presence was determined with D/max-2500 X-ray diffractometer(XRD). Quantitative energy dispersive spectroscopy(EDS) attached to SEM was used to measure the atom composition of points on the polished cross-sectional surface. Through comparing EDS results with Ni-Al binary phase diagram, phases were ascertained exactly[13].
3 RESULTS AND DISCUSSION
3.1 Microstructure of laminates
According to the phase diagram, there are some complex reactions and phase transformations in Ni-Al system. The transformations and diffusion reactions are different under different conditions, such as treating temperature and time. In fact, the phase transformations are determined by the reaction dynamics. According to the data in Ref.[14], the Gibbs free energy of possible reactions between Ni and Al are calculated and shown in Fig.4.
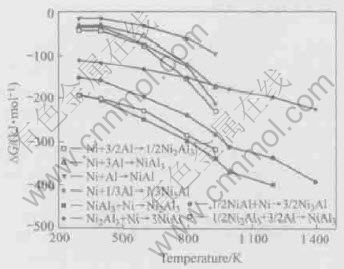
Fig.4 Relationship between Gibbs free energy and temperature of reactions
Among the direct reactions between Ni-Al atoms, the Gibbs free energy of the reaction Ni+3/2Al→1/2Ni2Al3 is the lowest. So Ni2Al3 phase should be the original phase in in-situ reaction, and the second phase NiAl3 should be generated by the reaction Ni2Al3+3Al→2NiAl3.
In order to prove the conclusion, the special experiment was designed additionally. In the experiment, the sample heated to 630℃ directly without pre-treatment for 30min and isothermally annealed for 20min, was scanned by SEM. The back scattering photo is shown in Fig.5. Due to the plastic deformation of Al foils during cold compressing, the interface between Ni-Al foils became rough, and the level of reaction was not uniform. Near the “peak top” of interface between Ni and Al foils, there exists only one phase Ni2Al3, contrariwise, two compound layers at the “valley bottom” as indicated in the figure.
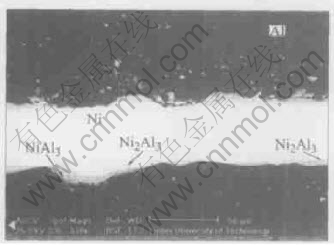
Fig.5 Primary phase and secondly produced phase
In order to avoid the high temperature oxidation, the samples of each level were treated at 300℃ for 90min before they were heated to 500℃, 630℃, 900℃ and 1100℃. The sectional photo of pre-treating only sample is shown in Fig.6. Though the total thickness of the compound layers is less than 1μm, the intermetallic layers bond the two kinds of foils tightly.

Fig.6 Back scattering photo of pre-treatment sample at 300℃
From the Ni-Al binary phase diagram, it can be found that the composition of NiAl3 phase is a fixed value, all the others have a composition range. So there must be a concentration transition region between Ni2Al3 and Al phase when the thickness of NiAl3 layer becomes big enough. According to the diffusion theory, there is never two-phase field during the diffusion reaction. Ni2Al3 is a kind of long-range order solid solution, and it can form under the appropriate external conditions. If the concentrations of Ni and Al atoms meet the suitable proportion in NiAl3 phase, Ni2Al3 forms and comes out of the NiAl3 phase(see Fig.7). The like “two-phase field” found in NiAl3 of 630℃ experiments can be considered many Ni2Al3 “islands” located in the “sea” of NiAl3. This phenomena also exits in 500℃ level specimens(see Fig.8).

Fig.7 Back scattering photo of 630℃/210min specimen

Fig.8 Back scattering photo of 500℃/180min specimen
At low reaction temperature, only two layers were generated between Ni and Al foils while the reactions lasted shortly(see Fig.5 and Fig.6). At 630℃, after the appropriate reaction time, the sample can present the typical layered structure referred in the Ref.[12](see Fig.3(b) and Fig.9 there). However, scanned by the back scattering in SEM, the typical compound layered structure can be distinguished more clearly, revealed in Fig.7 and Fig.9.
With the reaction continuing, the nickel concentration of compound layers will increase. Consequently, the high temperature phase NiAl, Ni3Al and even the Ni5Al3 appear near the Ni layer(see Fig.9). Their presence indicates that the key driving force of in-situ reaction is atom concentration, instead of the temperature.
With the increase of reaction temperature, the components of compound layer change accordingly. At 900℃ level, NiAl3 combines with Ni atoms and changes into Ni2Al3. Similarly, Ni2Al3 changes into NiAl at 1000℃ and 1100℃. The section microstructures of the three levels specimens are shown in Figs.10, 11 and 12, respectively. In fact, the vacuum in the experiments is not high (5×10-3Pa), and the high temperature intensifies the oxidation. The produced oxides are pushed at the growing interface and finally accumulated. Another influence of high temperature appears obviously in 1100℃ level specimens. Because of the large difference of elongation between Ni and the compound, the stress caused by the expand of Ni layers breaks the compound layers, where the cracks are arrowed in Fig.11. As a result, the tensile strength of the composites drops[13].

Fig.9 Back scattering photo of 630℃/600min specimen

Fig.10 Back scattering photo of 900℃/120min specimen
The XRD examination of total samples is shown in Fig.13. The composition of the points arrowed in figures were measured by EDS and listed in Table 1, respectively.

Fig.11 Back scattering photo of 1000℃/360min specimen

Fig.12 Back scattering photo of 1100℃/120min specimen
Ni5Al3 born between NiAl and Ni3Al layers is easy to decompose. The thickness of Ni5Al3 layer is small and nonuniform. Though its characteristic peaks can not be detected in XRD, it appears clearly in back scattering photo(Fig.9). And the EDS results also show its presence.
3.2 Growth of intermetallic compound layer
In different level experiments, either the dis tribution or the thickness of the compound layers is different. Though the compound layer consists of several different phases, all the phase transformations and growth of layers are controlled by atoms diffusion of Ni and Al. Because the interface between layers is plane, the diffusion process can be considered as one dimension. Any growth of interphase layer must be determined by the amount of atoms that diffuse through the existing compound layers[15]. Then Eqn.(1) holds.
dn=-dt·D ·q ·ΔC/y(1)
Table 1 Composition of points measured by EDS
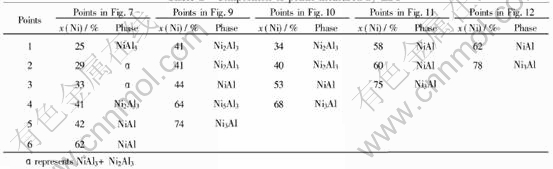
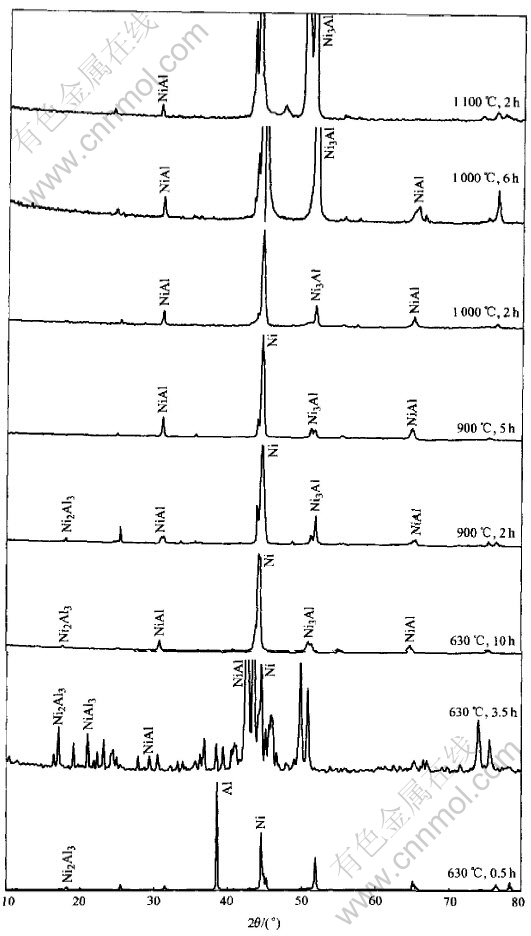
Fig.13 XRD patterns of samples
where dn is the amount of atoms passing through sectional area q during dt time, ΔC/y is the slope of the solute concentration along layer thickness y, D is diffusion coefficient. Because the value of ΔC/y in compound layer keeps constant, then
dn=a·q·dy(2)
where dy is the increase of layer thickness, a is a parameter that has dimension equal to concentration. Combining Eqn.(1) with Eqn.(2) yields
dt·D·ΔC/y=a·dy
Then
D·t·ΔC/a=y2/2
This is to say that the thickness of compound layer y is a function of t1/2, i.e. their growth follows exponential rule. In 630℃ experiments, due to the presence of the like “two phase field”, it is very difficult to separate the thickness of Ni2Al3 and NiAl3 phase independently. However, the thickness of the whole compound layer is recorded in Fig.14. Basically, the growth of compound layer coincides with the above equation.
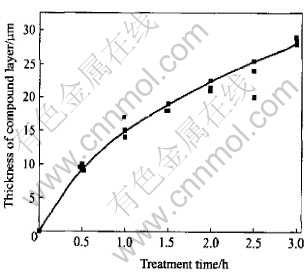
Fig.14 Relation between thickness of compound layer and treating time in 630℃ experiments
4 CONCLUSIONS
1) The high temperature phases NiAl and Ni3Al can be obtained under lower temperature treatment. It can be drawn that the key driving force of in-situ reactions is atom concentration, and temperature plays a subordinate role under certain conditions. The high temperature oxidation will also be avoided if decreasing the reaction temperature.
2) If the compound layer of laminated composite consists of NiAl3 phase, the microstructure of the laminated material is unstable. NiAl3 phase will combine with Ni atoms and transform into Ni2Al3 when the ambient temperature is near or higher than the treating temperature. The microstructure obtained at higher temperature or with long treating time is nevertheless comparatively stable. If the vacuum degree of equipment is improved, the treating temperature should increase accordingly.
3) The primary phase is Ni2Al3 during the diffusion reaction between Ni-Al. The original NiAl3 is transformed from Ni2Al3. When the diffusion of Ni and Al atoms continues at low temperature, the “two-phase field” appears consequently.
REFERENCES
[1]Stoloff N S, Liu C T. Emerging applications of intermetallics [J]. Intermetallics, 2000, 8(9): 1313-1320.
[2]Schulson E M. Hydrogen-boron interaction and its effect on the ductility and fracture of Ni3Al [J]. Scripta Materialia, 1998, 38(5): 845-846.
[3]Banerjee R, Thompson G B. Sputter deposited nanocrystalline Ni-25Al alloy thin films and Ni/Ni3Al multilayers [J]. Thin Solid Films, 2003, 424(1): 93-98.
[4]Ito S, Teiji Y. Machinability of Ni3Al-based intermetallic compounds [J]. Journal of Materials Processing, 1997, 63(1): 181-186.
[5]Brennan P C, Kao W G. Processing and heat treatment effects on an Al2O3/Ni3Al composite [J]. Materials and Manufacturing Processes, 1994, 9(2): 281-294.
[6]Phillipps A J, Clegg W J. Failure of layered ceramics in bending and tension [J]. Composites, 1994, 25: 524-533.
[7]HU J, LIN D L. Non-equilibrium grain boundary co-segregation of boron and magnesium in Ni3Al [J]. Acta Metall Sin, 2002, 38(8): 829-834.(in Chinese)
[8]Cermark J, Ruvzickova J. Influence of boron doping and stoichiometry upon the Ni grain boundary diffusion in Ni3Al intermetallic [J]. Scripta Materialia, 1997, 37(1): 31-35.
[9]GU Y F, LIN D L. High temperature deformation behaviors of directionally solidified Ni3Al alloy [J]. Acta Metall Sin, 1996, 32(11): 1144-1148.(in Chinese)
[10]ZHU P, LI C M, LIU C T. Combustion reaction in multilayered nickel and aluminum foils [J]. Material Sci Eng A, 1997, 239-240: 532-539.
[11]Mumtaz K, Echigoya J, Nakata C. Effect of cold rolling and subsequent annealing on hot pressed Ni/Al laminates [J]. Journal of Materials Science, 2001, 36: 3981-3987.
[12]XIA Z H, LIU J H. Fabrication of laminated metal-intermetallic composites by interlayer in-situ reaction [J]. Journal of Materials Science, 1999, 34(15): 3731-3735.
[13]ZHANG J, XIA Z H. Research of Ni-Al system metal/intermetallic compounds layered composite diffusion growth [J]. Journal of Hebei University of Technology, 1999, 28 (5): 36-39.(in Chinese)
[14]LIAN G, DONG Y J. Manual of Abiothermodynamics [M]. Shenyang: Northeastern University Press, 1993.(in Chinese)
[15]HE S A. Diffusion in Metal and Alloy [M]. Beijing: Science Press, 1958.(in Chinese)
(Edited by YUAN Sai-qian)
Received date: 2004-03-29; Accepted date: 2004-08-29
Correspondence: SUN Bao-de, Professor, PhD; Tel: +86-21-62834534; E-mail: bdsun@sjtu.edu.cn