ZrO2/TiO2复合光催化膜的微弧氧化法制备及表征
来源期刊:中国有色金属学报(英文版)2013年第10期
论文作者:罗 强 蔡启舟 李欣蔚 潘振华 李玉洁 陈喜娣 严青松
文章页码:2945 - 2950
关键词:纯钛;微弧氧化;Zr(OH)4;胶体颗粒;ZrO2/TiO2;复合光催化膜
Key words:pure titanium; micro-arc oxidation; Zr(OH)4; colloidal particle; ZrO2/TiO2; composite photocatalytic film
摘 要:采用微弧氧化方法和原位生成的Zr(OH)4胶体颗粒,在纯钛基体上制备ZrO2/TiO2复合光催化膜。采用SEM、EDX、XRD、UV-Vis DRS等分析手段,对膜层进行分析表征。结果表明:复合膜显示出层状和多孔的结构,由锐钛矿、金红石和ZrO2组成;相对于纯TiO2膜,复合膜层的光吸收截止边缘产生红移;ZnO2/TiO2复合膜层和纯TiO2膜层在紫外光照射下,对罗丹明B的光催化速率常数分别为0.0442和0.0186 h-1。
Abstract: ZrO2/TiO2 composite photocatalytic film was produced on the pure titanium substrate using in-situ Zr(OH)4 colloidal particle by the micro-arc oxidation technique and characterized by scanning electron microscope (SEM), energy dispersive X-ray (EDX), X-ray diffraction (XRD) and ultraviolet-visible (UV-Vis) spectrophotometer. The composite film shows a lamellar and porous structure which consists of anatase, rutile and ZrO2 phases. The optical absorption edge of film is shifted to longer wavelength when ZrO2 is introduced to TiO2. Furthermore, the photocatalytic reaction rate constants of degradation of rhodamine B solution with ZrO2/TiO2 composite film and pure TiO2 film under ultraviolet irradiation are measured as 0.0442 and 0.0186 h-1, respectively.
Trans. Nonferrous Met. Soc. China 23(2013) 2945-2950
Qiang LUO1, Qi-zhou CAI1, Xin-wei LI1, Zhen-hua PAN1, Yu-jie LI1, Xi-di CHEN1, Qing-song YAN2
1. State Key Laboratory of Material Processing and Dies and Mould Technology, Huazhong University of Science and Technology, Wuhan 430074, China;
2. National Defense Key Disciplines Laboratory of Light Alloy Processing Science and Technology, Nanchang Hangkong University, Nanchang 330063, China
Received 21 August 2012; accepted 30 January 2013
Abstract: ZrO2/TiO2 composite photocatalytic film was produced on the pure titanium substrate using in-situ Zr(OH)4 colloidal particle by the micro-arc oxidation technique and characterized by scanning electron microscope (SEM), energy dispersive X-ray (EDX), X-ray diffraction (XRD) and ultraviolet-visible (UV-Vis) spectrophotometer. The composite film shows a lamellar and porous structure which consists of anatase, rutile and ZrO2 phases. The optical absorption edge of film is shifted to longer wavelength when ZrO2 is introduced to TiO2. Furthermore, the photocatalytic reaction rate constants of degradation of rhodamine B solution with ZrO2/TiO2 composite film and pure TiO2 film under ultraviolet irradiation are measured as 0.0442 and 0.0186 h-1, respectively.
Key words: pure titanium; micro-arc oxidation; Zr(OH)4; colloidal particle; ZrO2/TiO2; composite photocatalytic film
1 Introduction
TiO2 photocatalysis is currently accepted as one of the most promising technologies for destruction of organic pollutions in the environment because it is cheap, chemically and biologically inert, non-toxic, photostable, and highly photo-reactive [1-4]. The immobilization of TiO2 is so critical for the practical photocatalytic application. Micro-arc oxidation (MAO) is a processing technique which can convert the surfaces of valve metals, such as aluminum, magnesium and titanium, into ceramic coatings [5-7]. The technique is considered to be appropriate for immobilizing the TiO2 photocatalyst, because the distinct properties of TiO2 formed via MAO include chemical durability, large specific surface area, remarkable thickness and good adhesion to metal substrate. Furthermore, the complex TiO2 film can be formed by adjusting electrolyte composition. There are only few reports on growing composite TiO2 photocatalytic films by MAO method. HE et al [8] prepared the WO3/TiO2 photocatalytic films on pure titanium substrate via MAO technique in tungstate electrolyte. BAYATI et al [9] used the MAO method to synthesize V2O5/TiO2 photocatalytic films. JIANG et al [10] fabricated the TiO2/YAG:Ce3+ compound photocatalytic films upon titanium alloy by micro-arc oxidation.
On the other hand, the fast recombination of photogenerated electron-hole pairs deteriorates the photocatalytic activity of TiO2, which limits the commercialization of this technology [11]. Some semiconductors have been used to couple with TiO2 in order to improve its photocatalytic activity. Among these semiconductors, ZrO2 is considered an appropriate semiconductor coupled with TiO2 because it is beneficial for the increase of surface area, the stabilization of anatase and the existence of stable electron-hole pairs [12-14]. As far as we know, ZrO2/TiO2 composite photocatalytic film has not been prepared by MAO technique to date. In this work, ZrO2/TiO2 composite photocatalytic film is prepared using in-situ Zr(OH)4 colloidal particle by MAO technique and the photocatalytic activity of the film is investigated.
2 Experimental
2.1 Preparation of film
The equipment used during the MAO process consists of an AC power supply, an electrolyte cell, a stirring system, a cooling system and an exhaust system. The pure titanium (99.5%) sheets with dimensions of 20 mm × 20 mm × 2 mm were polished by emery paper (150#-1200# grit), degreased by acetone and then rinsed by distilled water before the MAO process. The pure titanium sheet was selected as the anode and the stainless steel plate was used as the cathode. The electrolyte consisted of K2ZrF6 (7.1 g/L), NaOH (5 g/L) and NaF (1 g/L). In order to compare the microstructure and photophysical property of the ZrO2/TiO2 composite film with those of pure TiO2 film which was prepared in the electrolyte consisting of Na3PO4·12H2O (9.5 g/L), NaOH (1 g/L) and NaF (1 g/L). The electrolytes used to prepare ZrO2/TiO2 composite film and pure TiO2 film were marked as electrolytes A and B, respectively. The electrolyte temperature was kept under 40 °C through circulating water cooling system during the complete MAO process. The positive voltage, negative voltage, frequency, duty cycle and treatment time for both the ZrO2/TiO2 composite film and the pure TiO2 film were 300 V, -40 V, 700 Hz, 0.3 and 6 min, respectively. The as-prepared films were rinsed by distilled water, dried in hot air and then kept in a drying chamber.
2.2 Analysis of film
The phase composition and crystalline structure of films were determined by XRD analysis using a D/Max-IIIB diffractometer with Cu Kα radiation. A Quanta 200 scanning electron microscopy (SEM) was used to characterize the morphological feature of the films. The composition of films was analyzed with an energy dispersive X-ray (EDX) detector incorporated into the SEM. A Shimadzu UV-2550 UV-Vis spectrophotometer with an integrating sphere attachment recorded the diffuse reflectance spectra (DRS) of films.
2.3 Evaluation of photocatalytic activity
The photocatalytic activity of films was determined by degrading aqueous solutions of rhodamine B. The samples were submerged into 10 mL of rhodamine B solution (10 mg/L) in the dark for 30 min prior to irradiation in order to achieve adsorption/desorption equilibrium. An ultraviolet lamp (36 W) with a maximum irradiation peak at 254 nm was used as light source, the solution was constantly supplied with air during the irradiation process, a fixed quantity of solution was removed every 2 h to measure the absorbance. The absorbance of rhodamine B solution was tested at the wavelength of 550 nm with a UV-Vis spectrophotometer.
3 Results and discussion
3.1 XRD analysis
The XRD patterns of films prepared in different electrolytes are shown in Fig. 1. The characteristic peaks of Ti are detected from pure titanium substrates. A mixed phase of anatase, rutile and ZrO2 occurs in the ZrO2/TiO2 composite film. The mechanism for formation of ZrO2/TiO2 composite film can be interpreted as follows. At first, the reaction between K2ZrF6 and NaOH occurs in the electrolyte:
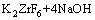 →
→ (1)
(1)
Zr(OH)4 is the colloidal particle, whose isoelectric point is 6.8, which means that Zr(OH)4 is electropositive when the pH of electrolyte is lower than 6.8 and Zr(OH)4 is electronegative when the pH of electrolyte is higher than 6.8 [15]. So, Zr(OH)4 colloidal particle should be electronegative in the alkaline electrolyte. When the MAO process begins, an oxidation reaction occurs on the surface of titanium substrate [16]:
Ti→Ti4++4e (2)
Meanwhile, the Zr(OH)4 particles and OH- anions move toward the anode surface because of the strong electrical field between anode and cathode, and then the following reactions occur because of the strong spark discharge on the surface of anode [16,17]:
Zr(OH)4→ZrO2+2H2O (3)
Ti4++4OH-→TiO2+2H2O (4)
The crystalline phase of the pure TiO2 film is anatase, because it is very difficult for the metastable anatase phase to transform to thermodynamically stable rutile phase due to less heat produced by mild spark discharge on the surface of anode. The ratio of rutile to anatase in the ZrO2/TiO2 composite film is very high. Furthermore, the photocatalytic activity of rutile is lower than that of anatase. At the same time, because the conductive band of anatase phase locates at a higher energy position than that of rutile phase by about 0.20 eV, the photo-generated electron can transfer from rutile to anatase, which effectively inhibits the recombination of photo-generated electron-hole pairs [18,19]. Therefore, the ZrO2/TiO2 composite film should have a higher photonic efficiency compared with pure TiO2 film.

Fig. 1 XRD patterns of films prepared in different electrolytes
3.2 Microstructures
The surface micrographs of films prepared in different electrolytes are shown in Fig. 2. Obviously, the surface of ZrO2/TiO2 composite film exhibits a lamellar and porous microstructure. Furthermore, on the surface of film, there are many bright particles which are agglomerated. The EDX analysis of the bright particle marked in Fig. 2(b) is shown in Fig. 3, and the contents of elements are presented in Table 1. The bright particle mainly contain 22.95% Zr, 11.13% Ti and 65.91% O (mole fraction), which is similar to the chemical compositions of the mixture of ZrO2 and TiO2. The holes in the film are not only the reaction channels between electrolyte and substrate but also the channels through which molten oxide ejects during the MAO process. The strong spark discharge on the surface of anode generates high temperature and pressure, which results that the titanium substrate melts and then enters into the reaction channels, and is oxidized. The oxidized titanium rapidly ejects from the channels and solidifies because of the cold quenching of electrolyte. Meanwhile, the Zr(OH)4 particles translate to the ZrO2 particles on the surface of anode and then combine with the oxidized titanium to form the mixture of ZrO2 and TiO2. Although the surface of pure TiO2 film also exhibits a porous microstructure, the surface of pure TiO2 film is relatively smooth compared with the ZrO2/TiO2 composite film. Mild spark discharge means fewer discharge channels on the surface of pure TiO2 film compared with the ZrO2/TiO2 composite film, which results that the surface of pure TiO2 film is relatively smooth compared with the ZrO2/TiO2 composite film.

Fig. 2 Surface micrographs of films prepared in different electrolytes
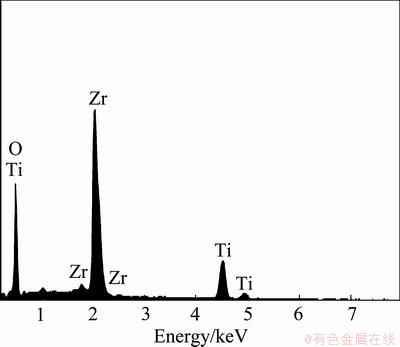
Fig. 3 EDX analysis corresponding to point A in Fig. 2(b)
Table 1 Contents of surface element of bright particles

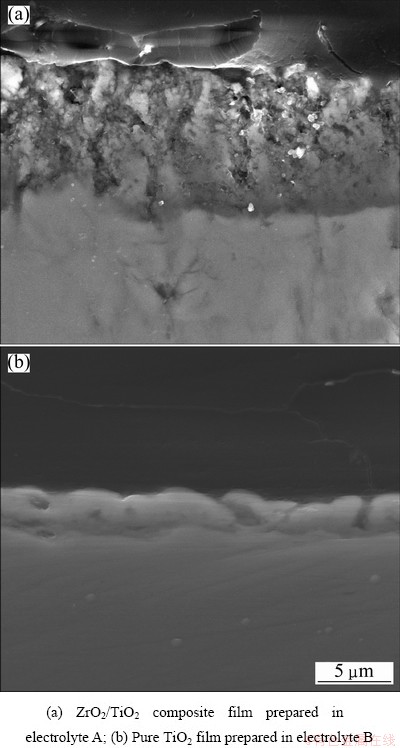
Fig. 4 Cross-section micrographs of films prepared in different electrolytes
The cross-section micrographs of films prepared in different electrolytes are shown in Fig. 4. There is no obvious discontinuity between the films and the substrate, which proves that the films are well adhered to the titanium substrate. This is beneficial to long-term degradation of organic compounds. However, there are two considerable differences in cross-section micrographs of two films. The thicknesses of the ZrO2/TiO2 composite film and the pure TiO2 film are approximately 10 and 3 μm, respectively. Furthermore, compared with the pure TiO2 film, there are many micro-pores in the inner layer of the ZrO2/TiO2 composite film. The growth of the MAO film is the result of melting and solidifying of oxide through the discharge channels, therefore, more discharge channels mean faster growth rate and the ZrO2/TiO2 composite film should be thicker than the pure TiO2 film during the same period.
The differences of the surface and cross-section morphologies between the two films are attributed to more violent spark discharge during the MAO process of ZrO2/TiO2 composite film. For ZrO2/TiO2 composite film, the lamellar and porous microstructure, agglomerated ZrO2/TiO2 mixed particles and micro-pores in the inner layer of film should be responsible for providing more surface sites, in which more organic compounds and photons can be absorbed compared with the pure TiO2 film.
3.3 UV-Vis absorption spectra
The UV-Vis absorption spectra of films prepared in different electrolytes are shown in Fig. 5. Obviously, the optical absorption edges of the ZrO2/TiO2 composite film and the pure TiO2 film are about 421 and 412 nm, respectively. A slight shift towards longer wavelength in absorption band is detected when ZrO2 is introduced to TiO2. This behavior is in a good agreement with achievement of Ref. [20]. Zr ions may enter into the lattice of TiO2 because of violent spark discharge during the MAO process, which leads to the lattice deformation as reported by WANG et al [21]. Therefore, some structural defects such as vacancies in the lattice would be produced particularly on the surface to partially offset the lattice strain [22]. The enhanced concentration of the oxygen vacancies in binary oxides system forms a defect band that further changes the energy of conduction band [23]. However, the ZrO2/TiO2 composite film exhibits lower absorption in the visible light region compared with the pure TiO2 film, because ZrO2 has a lower ability of absorbing visible light compared with TiO2 and the excessive ZrO2 can cover the surface of composite films, resulting that very little visible light is absorbed by ZrO2/TiO2 composite film.
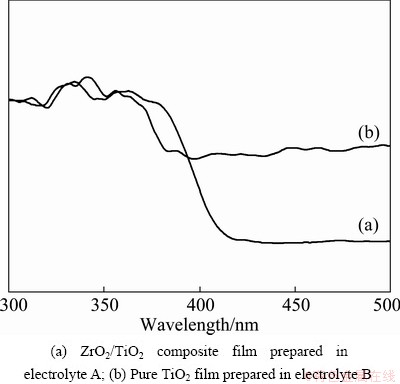
Fig. 5 UV-Vis absorption spectra of films prepared in different electrolytes
3.4 Photocatalytic activity
No detectable degradation of rhodamine B occurs without MAO films under UV light radiation. It is proved that the photocatalytic decomposition of rhodamine B solution agrees with the apparent first-order model [24]:
lnC0/C=kt (5)
where C0 is the initial concentration of the rhodamine B solution at t=0; C is the concentration of the rhodamine B solution at latter time t; t is the irradiation time and k is the reaction rate constants. Figure 6 illustrates the relationship of ln C0/C versus irradiation time for two films. The photocatalytic reaction rate constants of degradation of rhodamine B solution with ZrO2/TiO2 composite film and pure TiO2 film under UV irradiation are measured as 0.0442 and 0.0186 h-1, respectively. A comparison between the photocatalytic activities of the two films emphasizes the photocatalytic enhancement in the ZrO2/TiO2 composite film, which can be mainly attributed to the mixed phase, more surface sites and formation of structural defects. The ZrO2/TiO2 composite film has the mixed phase of anatase, rutile and ZrO2, which results that the photo-generated electron can transfer from rutile to anatase. This effectively inhibits the recombination of photo-generated electron-hole pairs. The lamellar and porous microstructure, agglomerated ZrO2/TiO2 mixed particles and micro-pores in the inner layer should be responsible for providing more surface sites, in which more absorbed organic compounds can be degraded by OH-. Furthermore, some oxygen might be escaped from the surface of the lattice to trap the photo-generated holes because of the formation of vacancies on the surface [22]. In addition, metal oxides with more structure defects on surface are able to substantially ionosorb oxygen, in the form of O2- species, which are involved in electron capture in aqueous phase reactions, to let photoholes react with surface OH- groups [25]. Another reason of the enhanced photocatalytic activity is zirconia modified titania with higher surface acidity, the surface sites with higher acidity may prove to be better adsorption sites or hole traps [20].
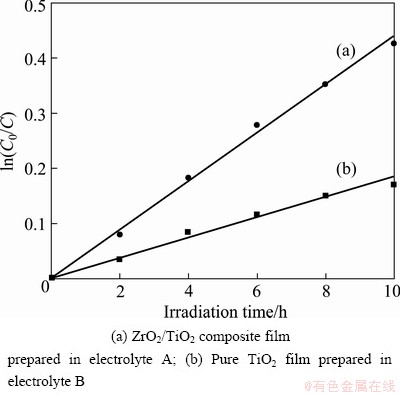
Fig. 6 Relationship of ln C0/C versus irradiation time for films prepared in different electrolytes
4 Conclusions
ZrO2/TiO2 composite film with a lamellar and porous morphology was successfully produced in the electrolyte consisted of in-situ Zr(OH)4 colloidal particle by the MAO technique. The composite film consists of anatase, rutile and ZrO2 phases. The optical absorption edges of the ZrO2/TiO2 composite film and the pure TiO2 film are about 421 and 412 nm, respectively. It is also revealed that the photocatalytic activity of ZrO2/TiO2 composite film enhances approximate three times compared with pure TiO2 film under UV irradiation. Future works will be focused on the further improvement of photocatalytic activity of ZrO2/TiO2 composite film under ultraviolet and visible irradiations by adjusting process parameters, such as power voltage, reaction time and electrolyte composition.
References
[1] POPA M, DIAMANDESCU L, VASILIU F, TEODORESCU C M, COSOVEANU V, BAIA M, FEDER M, BAIA L, DANCIU V. Synthesis, structural characterization, and photocatalytic properties of iron-doped TiO2aerogels [J]. Journal of Materials Science, 2009, 44(2): 358-364.
[2] HOFFMANN M R, MARTIN S T, CHOI W, BAHNEMANN D W. Environmental applications of semiconductor photocatalysis [J]. Chemical Reviews, 1995, 95(1): 69-96.
[3] CHEN Hua-wei, KU Young, KUO Yu-lin. Effect of Pt/TiO2 characteristics on temporal behavior of o-cresol decomposition by visible light-induced photocatalysis [J]. Water Research, 2007, 41(10): 2069-2078.
[4] KOSOWSKA B, MOZIA S, MORAWSKI A W, GRZMIL B, JANUS M, KALUCKI K. The preparation of TiO2-nitrogen doped by calcination of TiO2xH2O under ammonia atmosphere for visible light photocatalysis [J]. Solar Energy Materials and Solar Cells, 2005, 88(3): 269-280.
[5] LU Li-hong, SHEN De-jiu, ZHANG Jing-wu, SONG Jian, LI Liang. Evolution of micro-arc oxidation behaviors of the hot-dipping aluminum coatings on Q235 steel substrate [J]. Applied Surface Science, 2011, 257(9): 4144-4150.
[6] WANG Ping, LI Jian-ping, GUO Yong-chun, YANG Zhong. Growth process and corrosion resistance of ceramic coatings of micro-arc oxidation on Mg-Gd-Y magnesium alloys [J]. Journal of Rare Earths, 2010, 28(5): 798-802.
[7] KIM M S, RYU J J, SUNG Y M. One-step approach for nano-crystalline hydroxyapatite coating on titanium via micro-arc oxidation [J]. Electrochemistry Communications, 2007, 9(8): 1886-1891.
[8] HE Jian, CAI Qi-zhou, JI Y G, LUO H H, LI D J, YU B. Influence of fluorine on the structure and photocatalytic activity of TiO2 film prepared in tungstate-electrolyte via micro-arc oxidation [J]. Journal of Alloys and Compounds, 2009, 482(1-2): 476-481.
[9] BAYATI M R, MOSHFEGH A Z, GOLESTANI-FARD F. Synthesis of narrow band gap (V2O5)x-(TiO2)1-x nano-structured layers via micro arc oxidation [J]. Applied Surface Science, 2010, 256(9): 2903-2909.
[10] JIANG Xu-dong, WANG Yong-qian, PAN Chun-xu. Micro-arc oxidation of TC4 substrates to fabricate TiO2/YAG: Ce3+ compound films with enhanced photocatalytic activity [J]. Journal of Alloys and Compounds, 2011, 509(8): L137-L141.
[11] XU Zi-li, YANG Qiu-jing, XIE Chao, YAN Wei-jun, DU Yao-guo, GAO Zhong-min, ZHANG Jia-hua. Structure, luminescence properties and photocatalytic activityofeuropium doped-TiO2 nanoparticles [J]. Journal of Materials Science, 2005, 40(6): 1539-1541.
[12] MCMANAMON C, HOLMES J D, MORRIS M A. Improved photocatalytic degradation rates of phenol achieved using novel porous ZrO2-doped TiO2 nanoparticulate powders [J]. Journal of Hazardous Materials, 2011, 193: 120-127.
[13] HIRANO M, NAKAHARA C, OTA K, TANAIKE O, INAGAKI M. Photoactivity and phase stability of ZrO2-doped anatase-type TiO2 directly formed as nanometer-sized particles by hydrolysis under hydrothermal conditions [J]. Journal of Solid State Chemistry, 2003, 170(1): 39-47.
[14] HERNANDEZ-ALONSO M D, TEJEDOR-TEJEDOR I, CORONADO J M, SORIA J, ANDERSON M A. Sol–gel preparation of TiO2-ZrO2 thin films supported on glass rings: Influence of phase composition on photocatalytic activity [J]. Thin Solid Films, 2006, 502(1-2): 125-131.
[15] XU Yu-fen, FAN Wen-yuan. Research on separating Zr(OH)4 suspension with ceramic microfiltration membranes [J]. China Powder Science and Technology, 2000, 6: 251-253. (in Chinese)
[16] BAYATI M R, MOSHFEGH A Z, GOLESTANI-FARD F. In situ growth of vanadia-titania nano/micro-porous layers with enhanced photocatalytic performance by micro-arc oxidation [J]. Electrochimica Acta, 2010, 55(9): 3093-3102.
[17] LUO Hai-he. Preparation, characterization and performances of ZrO2-Y2O3-containing composite ceramic coatings on AZ91D magnesium alloy by microarc oxidation [D]. Wuhan: Huazhong University of Science and Technology, 2009: 57. (in Chinese)
[18] BICKLEY R I, GONZALEZ-CARRENO T, LEES J S, PALMISANO L, TILLEY R J D. A structural investigation of titanium dioxide photocatalysts [J]. Journal of Solid State Chemistry, 1991, 92(1): 178-190.
[19] SHI Lei, WENG Duan. Highly active mixed-phase TiO2 photocatalysts fabricated at low temperature and the correlation between phase composition and photocatalytic activity [J]. Journal of Environmental Sciences, 2008, 20(10): 1263-1267.
[20] WU Bao-chao, YUAN Ru-sheng, FU Xian-zhi. Structural characterization and photocatalytic activity of hollow binary ZrO2/TiO2 oxide fibers [J]. Journal of Solid State Chemistry, 2009, 182(3): 560-565.
[21] WANG Yan-min, LIU Su-wen, LU Meng-kai, WANG Shu-fen, GU Feng, GAI Xue-zhou, CUI Xiao-peng, PAN Jie. Preparation and photocatalytic properties of Zr4+-doped TiO2 nanocrystals [J]. Journal of Molecular Catalysis A: Chemical, 2004, 215(1-2): 137-142.
[22] YU J C, LIN J, KWOK R W M. Ti1-xZrxO2 Solid solutions for the photocatalytic degradation of acetone in air [J]. Journal of Physical Chemistry B, 1998, 102(26): 5094-5098.
[23]  P, BAKARDJIEVA S,
P, BAKARDJIEVA S,  J. Influence of Zr as TiO2 doping ion on photocatalytic degradation of 4-chlorophenol [J]. Applied Catalysis B: Environmental, 2007, 74(1-2): 83-91.
J. Influence of Zr as TiO2 doping ion on photocatalytic degradation of 4-chlorophenol [J]. Applied Catalysis B: Environmental, 2007, 74(1-2): 83-91.
[24] LIU Guo-guang, ZHANG Xue-zhi, XU Ya-jie, NIU Xin-shu, ZHENG Li-qing, DING Xue-jun. Effect of ZnFe2O4 doping on the photocatalytic activity of TiO2 [J]. Chemosphere, 2004, 55(9): 1287-1291.
[25] SCLAFANI A, HERRMANN J M. Comparison of the photoelectronic and photocatalytic activities of various anatase and rutile forms of titania in pure liquid organic phases and in aqueous solutions [J]. Journal of Physical Chemistry, 1996, 100(32): 13655-13661.
罗 强1,蔡启舟1,李欣蔚1,潘振华1,李玉洁1,陈喜娣1,严青松2
1. 华中科技大学 材料成形与模具技术国家重点实验室,武汉 430074;
2. 南昌航空大学 轻合金加工科学与技术国防重点学科实验室,南昌 330063
摘 要:采用微弧氧化方法和原位生成的Zr(OH)4胶体颗粒,在纯钛基体上制备ZrO2/TiO2复合光催化膜。采用SEM、EDX、XRD、UV-Vis DRS等分析手段,对膜层进行分析表征。结果表明:复合膜显示出层状和多孔的结构,由锐钛矿、金红石和ZrO2组成;相对于纯TiO2膜,复合膜层的光吸收截止边缘产生红移;ZnO2/TiO2复合膜层和纯TiO2膜层在紫外光照射下,对罗丹明B的光催化速率常数分别为0.0442和0.0186 h-1。
关键词:纯钛;微弧氧化;Zr(OH)4;胶体颗粒;ZrO2/TiO2;复合光催化膜
(Edited by Chao WANG)
Foundation item: Project (gf200901002) supported by the Open Research Fund of National Defense Key Disciplines Laboratory of Light Alloy Processing Science and Technology of Nanchang Hangkong University, China
Corresponding author: Qi-zhou CAI; Tel/Fax: +86-27-87558190; E-mail: caiqizhou@mail.hust.edu.cn
DOI: 10.1016/S1003-6326(13)62818-6

