
Direct electroless Ni-P plating on AZ91D magnesium alloy
LI Zhong-hou(李忠厚)1,QU Yu-ping(渠毓萍)1,ZHENG Feng(郑 峰)2,DAI A-gan(戴阿赶)2
1. Research Institute of Surface Engineering, Taiyuan University of Technology, Taiyuan 030024; China;
2. Shanxi Seikou Institute of Magnesium Technology, Taiyuan 030002, China
Received 28 July 2006; accepted 15 September 2006
Abstract: An electroless Ni-P plating treatment was applied on AZ91D magnesium alloy to improve its corrosion resistance. Optimum pretreatment conditions and optimum bath of electroless nickel plating for magnesium alloy were found through many experiments. In order to avoid bother of pre-plating medium layer, a set of procedure of direct electroless Ni-P under the acid condition was investigated. The properties of the coating with 10% phosphorus were investigated. The results show that a coating with high hardness, low porosity and good adhesive strength is obtained. X-ray diffraction patterns show that the structure of the coating is an amorphous phase. After annealing at 400 ℃, the amorphous phase of Ni-P is transformed to crystalline phases,and some intermetallics as Ni3P and Ni5P2 are deposited from Ni –P solid solution along with an enhancing hardness from Hv 450 to Hv 910.
Key words: magnesium alloy; direct electroless Ni-P plating; amorphous; adhesive strength
1 Introduction
Magnesium and its alloys with one quarter of the density of steel and only two thirds of that of aluminium and high specific strength[1-3] were used in telecommunication and aerospace as an ultra light mass alloy. At same time, magnesium and its alloys possessed many excellent processing properties, such as excellent castability, machinability and damping characteristic[4,5], hence, these alloys were also elected in automobile, motorcycle, airplane applications, etc. However, magnesium is an intrinsiccally highly reactive (standard potential –2.37 V), it is prone to atmospheric corrosion, which is actually one of the main obstacles to the application of magnesium alloy in practical fields. Some sorts of surface engineering techniques should be applied to magnesium alloys in order to meet some practical applications.
Various surface treatment, chemical conversion, anode oxidation, painting and electroplating for magnesium alloys were investigated extensively, but a great improvement of corrosion resistance and wear resistance of magnesium alloys could not be gained by chemical conversion, anode oxidation and painting[6]. The electroplating is mainly conducted with cyanide and CrO3 or chromate solutions[7], hence, this treatment is harmful to environment. Electroless nickel plating on magnesium alloys is a proper protection method because the deposited nickel is very resistant to corrosion and abrasions and this treatment is environment-friendly. Being a highly active metal, electroless plating of magnesium alloy needed special bath formulations and special pre-treatment procedures. The complex pre-treatment involved pre-plating medium layer as plating copper and plating zinc[8].In pre-plating copper and zinc process, the plating solution contained some cyanides and CrO3. Hence, a direct electroless plating process of magnesium alloy was put forward to escape from foregoing demerits[1-2]. Because of high electrochemical activity of magnesium, depositing coating on the surface of magnesium alloy was still a challenge by direct electroless plating for the researchers.
In this study, a set of procedure of direct electro- less Ni-P under the acid condition was developed for AZ91D alloy to improve its corrosion resistance. The microstructures and corrosion behaviour of the nickel coating was studied in detail.
2 Experimental
The specimens with dimension of 15 mm×15 mm×10 mm were cut from sand-cast AZ91D magnesium alloy. The pre-treated solution and the plating solution were confected with the chemical agents of the chemical purity and deionized water.
The sequence of steps of the electroless Ni-P plating were as follows: pre-abraded samples; chemical degrease and hot water rinse after cooled water rinse; acid pickling and cooled water rinse; activation and hot water rinse after cooled water rinse; electroless Ni-P plating and hot water rinse; evaporation. The surfaces of the specimens were mechanically polished by emery paper up to 1 000 grit to ensure that all samples get the same surface roughness before experiment. The purpose of degrease was cleaning the oil dirt of surface on the specimen, so that the surface became hydrophile. The composition of degrease bath and operation condition are shown in Table 1.
Table 1 Composition of degrease bath and operation condition

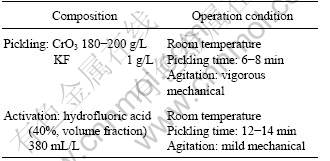
The purpose of pickling was to dissolve the oxide of the surface of specimens by chemical reaction. The specimens were quickly transferred to activation bath after pickling and water rinse. The residual oxide film and the leftover after pickling on the surface of the specimen were wiped away ulteriorly in the activation bath. The components of the acid pickling bath and the activation bath and operation conditions were found through many experiments, as shown in Table 2.
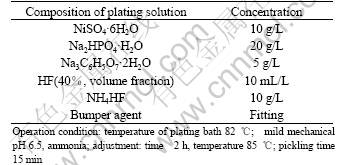
The specimens after pre-treatment were quickly transferred to electroless bath for electroless Ni-P plating. Composition of the plating bath and operation condition were also found through many experiments(as shown in Table 3).
Table 2 Composition of acid pickling bath and activation bath and operation condition
The temperature of the electroless plating bath was controlled by immersing the plating cell in a constant temperature water bath.
The composition of coating was measured by glow discharge spectrometry(GDS). The adhesive strength of the coating and substrate was estimated by the heat shock and the nick experiments. The microstructures and the constituent phases of the coating were determined by Axiovert model optical microscope with computer analyzing software and RigakuD/max 2500 model X-ray spectrometer before and after heat treatment. The hardness of the coating was measured by Leco M-400-H1 microhardness tester. The porosity existed in the coating was measured by pasted paper with chemical agent.
Table 3 Composition of plating solution and operation condition
3 Results and discussion Fig.1 shows the light micrograph of cross section after electroless Ni-P plating for AZ91D magnesium alloy. From Fig.1, the microstructure of AZ91D ingot is consisted of α solid solution and β-phase. β-phase is an intermetallic with stoichiometric composition of Mg17Al12 and locates on grain boundaries of α phase. After the direct electroless plating, an even and compact coating is formed onto the AZ91D magnesium alloy. The thickness of the coating is about 30 μm after electroless plating at 82 ℃ for 2 h.
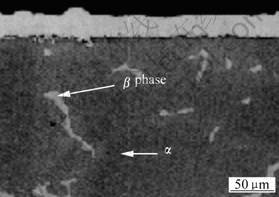
Fig.1 Light micrograph of cross section after electroless-plating Ni-P for AZ91D magnesium alloy
The composition of coating was measured by glow discharge spectrometry(GDS). Fig.2 shows the elements contribution of the electroless plating Ni-P layer. The result shows that the phosphorous content of the coating reaches 10%(mass fraction), and elements of both side at the interface occur commutative diffusion under condition of the electroless plating. The width of the diffusion zone reaches 15 μm or so. Without question, the existing of diffusion zone will increase the adhesive strength between the coating and the substrate. The heat shock experiment is a method that appraises qualitatively the adhesive strength. The specimens were heated for 1 h at elected temperature and then quickly were transferred to a cool water bath for judging its adhesive strength through examining peeling off. The result is shown in Table 4. It is obvious that a good adhesive strength between the coating and substrate is obtained after electroless Ni plating. The nick experiment is an other method of estimating the adhesive strength. When a diamond pyramid cuts vertically in the coating and ploughs ahead, in which the cut-in force increases with the increase of moving distance of diamond pyramid, and if cracks between the coating and substructure come into being, a sound signal can be shown on the display screen of computer. The larger the load of sound signal appeared firstly is, then, the stronger the linking of them is. The sound emission curve of the coating was determined by WS-97 model nick instrument, and the results are shown in Fig.3. Before vertical force of the diamond pyramid reaches 65 N, no sound emission is observed, which testifies a stronger binding between them. The surface nick morphology of the coating is observed by Axiovert model optical microscope with computer analyzing software, and the results are shown in Fig.4. From Fig.4, no peeling or crack is observed in edge of the nick, which testifies also a firm link of them.
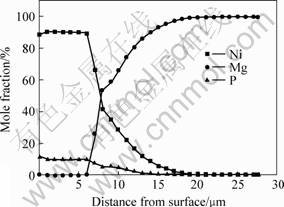
Fig.2 Composition distribution of electrolessplating Ni-P layer
Table 4 Heat shock experiment for adhesive strength of coatings and substrate
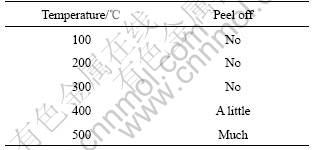
Fig.3 Sound emission curve of nick experiment for plating layer
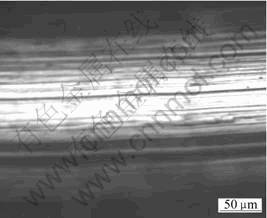
Fig.4 Surface morphology of electroless Ni plating layer after nick experiment
Fig.5(a) shows that the coating formed under acid condition is amorphous possibly with microcrystalline areas. The broad peak in the diffractogram corresponds to the dominant peak (111)of nickel. Fig.5(b) shows that some intermetallics, such as Ni3P and Ni5P2, are deposited from solid solution of Ni-P, when coating was tempered at 400 ℃ for 1 h. The most important effect of precipitation of the second phase is that the coating is hardened, its hardness increases to 910 HV0.2 from 450 HV0.2 of plating state.
The porosity existed in the coating was measured by pasted paper with chemical agent. The finally evaluated porosity of the coating is the average of three experiments, which reduces occasional data errors. The results show that the coating has low porosity, less than one in per square centimeter. As the nickel coating is cathodic relative to the substrate of magnesium, pin hole penetrated through substrate can arise from the catastrophic galvanic corrosion between nickel and magnesium alloy. Hence, the coating with low porosity is required for corrosion resistance of magnesium alloy.
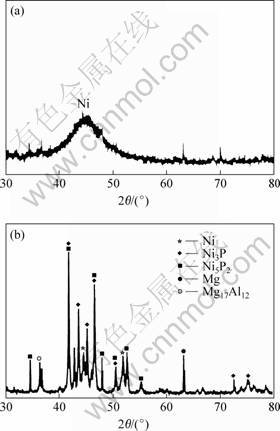
Fig.5 XRD patterns of constituent phases of coating: (a) Plating state; (b) Tempered at 400 ℃ 4 Conclusions 1) A commutative diffusion zone between the coating and substrate will be beneficial to increasing binding strength.
2) The adhesive strength of coating and substrate is very good after direct electroless plating.3) Low porosity of the coating is obtained, which is less than one in per square centimeter.
4) The structure of coating is amorphous possibly with microcrystalline areas in acid bath .When tempering at 400 ℃, some intermetallics, such as Ni3P and Ni5P2, are deposited from Ni-P solid solution , as a result, the hardness of the coating increases greatly.
References
[1] SHARMA A K, SURESH M R, BHOJRAJ H, NARAYANAMURTHY H, SAHU R P. Electroless nickel plating on magnesium alloy[J]. Metal Finishing,1998, 96: 10-18.
[2] AMBAT R, ZHOU Wei. Electroless nickel-plating on AZ91D magnesium alloy: Effect of substrate microstructure and plating parameters[J]. Surface and Coating Technology, 2004, 179: 124-134.
[3] HUO Hong-wei, LI Ying, WANG Fu-hui. Corrosion of AZ91D magnesium alloy with a chemical conversion coating and electroless nickel layer[J]. Corrosion Science, 2004, 46: 1467-1477.
[4] GU Chang-dong, LIAN Jian-she, HE Jin-guo, JIANG Zhong-hao, JIANG Qing. High corrosion-resistance nanocrystalline Ni coating on AZ91D magnesium alloy[J]. Surface and Coatings Technology, 2006, 200: 5413-5418.
[5] GRAY J E, LUAN B. Protective coating on magnesium and its alloys-a critical review[J]. Journal of Alloys and Compounds, 2002, 336: 88-113.
[6] ZHANG Yong-jun, YAN Chuan-wei, WANG Fu-hui, LOU Han-yi. Environmental friendly bath solution and process for anodization of magnesium and it’s alloys[J]. Materials Protection, 2002, 35(3): 35-39.
[7] BRUNELLI K, DABALA M, CALLIARI I, MAGRIN M. Effect of HCL pre-treatment on corrosion resistance of cerium-based conversion coating on magnesium and magnesium alloys[J]. Corrosion Science, 2005, 47: 989-1000.
[8] LUO Shou-fu, HU Wen-bin. Electroless nickel plating of aluminium and magnesium alloys[J]. Process Technology of Light Alloys, 1997, 25( 2): 28-29.
(Edited by LI Yan-hong)
Foundation item: Project (2006031117-04) supported by the Key Technique Item of Shanxi Province, China
Corresponding author: LI Zhong-hou; Tel: +86-z 351-6010540; E-mail: lzh8085217@21cn.com