文章编号:1004-0609(2007)01-0172-07
金、银和铜氰化溶解速率及硫离子对其影响的比较
王 宇,陈 景,韦群燕,谢琦莹
(云南大学 化学系,昆明 650091)
摘 要:用旋转圆盘法在相同的实验条件下,对金、银、铜的氰化溶解速率进行比较研究。当氰化钠浓度为5.00 g/L、圆盘转速为600 r/min、温度为30 ℃时,获得表观速率常数的顺序为:Au<Ag<Cu。在氰化钠溶液中加入微量硫化钠后,硫离子抑制氰化反应的影响程度则为:Au>Ag>Cu。分析讨论了电化学反应机理,提出了相应的观点并对实验结果进行了解释。
关键词:金;银;铜;氰化溶解;硫离子
中图分类号:0643.12 文献标识码:A
Comparison of cyanidation rates of gold, silver and copper and influences of sulfion on them
WANG Yu, CHEN Jing, WEI Qun-yan, XIE Qi-ying
(Department of Chemistry, Yunnan University, Kunming 650091, China)
Abstract: The cyanidation rates of gold, silver and copper were compared with each other under the same reaction conditions by kinetic rotating disk method, and the influences of sulfion on them were discussed. The results show that when the three metals react at ρ(NaCN) of 5.00 g/L, 600 r/min and 30 ℃, the obtained apparent rate constants for cyanide dissolution of three metals accord with the order of Au<Ag<Cu. However, when sodium sulfide is added into the cyanide solutions in advance, the decrease ratios of cyanide dissolution rates of the three metals accord with the order of Au>Ag>Cu. The reaction thermodynamics and mechanism based on the electrochemical theory were discussed, and some new explanations for experimental phenomena were proposed.
Key words: gold; silver; copper; cyanidation; sulfion
19世纪末,用氰化钠溶液从金矿石中提取金的方法被应用于黄金的工业生产,这是湿法冶金发展史上的一座里程碑。100多年以来,90%以上的矿产金都是用氰化法提取。氰化法提取金在现代黄金生产中仍然居统治地位。
对于氰化物溶金反应的热力学和动力学,人们已进行相当深入地研究。早期的研究偏重在热力学计算确定氰化溶金的反应式,计算反应平衡常数以及绘制φ—pH图。动力学研究则在于了解溶液中的氧浓度、氰根浓度、金的总面积、搅拌速度以及温度等因素对反应速率的影响。对于反应机理,从19世纪中期起,曾出现过氧论、氢论、过氧化氢论、氰论、腐蚀论和电化学溶解论[1],比较公认的是电化学过程[2]。阳极反应是金以配离子形式进入溶液并释放出等当量的电子;阴极反应是溶解在水中的氧作为去极剂夺取过剩的电子。对于阳极反应文献中有两种写法,如在文献[3-4]中为

两种写法的差别在于是否存在先形成Au+的过程。
近年来,Wadsworth等[7-8]用电化学方法研究金的溶解机理,用晶体表面传质伴随电荷传输的过程解释了所获得的实验数据,对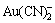 配离子的形成过程又提出了新的观点。
配离子的形成过程又提出了新的观点。
银常伴生在金矿物中,银的氰化反应也被广泛的研究过。但对于氰化溶解银和溶解金的反应速率比 较,文献中说法不一。如文献[3]认为,在扩散控制范围内,“在同样条件下,金和银应以同一速度溶解”。在文献[1,5]引用的氰化液浓度与金银氰化溶解量的图中,金的氰化溶解速率快于银。只有文献[9]认为,根据原电池电动势的大小,可以排列出金属氰化由 易到难的顺序为:铜、银、金、钯,而铂不能被氰化。但是,标准电极电位值属热力学数据,其他文献报道的金银的氰化溶解速率顺序并不符合标准电位值顺序。
从化学性质来看,地质学家还认为金原子由于受相对论效应的影响,在矿床形成的过程中,金可与S2-、SH-、CN-等负离子形成可迁移的配合物,甚至比铜、铅、锌还不稳定[10-12]。
对于铜的氰化,文献[1,3,13]均认为,铜矿物和金属铜都是耗氰物质,金矿石中存在千分之几的铜,都会造成氰化物大量消耗,通常铜含量应控制在0.1%以下,但未查到氰化溶解铜动力学研究的报道。
金、银、铜同属周期表中的IB族,原子的外电子结构相似,化学性质相近,其氰化反应可能随周期数变化。但文献中金、银、铜氰化反应的热力学计算、动力学速率顺序以及反应机理的观点比较混乱,因此在同等条件下,特别是在化学反应控制区,对氰化溶解速率进行比较研究,深化对反应过程的认识,将具有一定的学术意义。此外,已知在氰化溶金的反应过程中,10-6量级的硫离子(S2-)即可显著地抑制金的氰化。因此,生产中要求硫离子要低于0.05×10-6 [14]。但文献中缺乏硫离子对银、铜氰化速率影响程度的报道。因此本文作者对此问题也进行了比较研究,并定性地讨论了硫离子对银、铜氰化速率的影响机理。
1 实验
旋转圆盘设备用无极调速的小电机自行组装,转速可精确控制,并用数字显示,转轴及固定金属圆片的螺栓使用聚四氟乙烯材质。金、银、铜片材纯度均为99.99%,厚度为 0.5 mm,剪成外径18 mm,内径5 mm的圆环,用螺栓拧在搅拌轴上。反应器为容积200 mL的玻璃夹套烧杯。
先配制pH值为10.5,浓度为50.0 g/L的氰化钠贮备液。配制1 g/L的硫化钠储备液。取稀释到预定浓度的氰化钠溶液150 mL于玻璃夹层反应器中,用超级恒温水浴锅的外循环水使夹层反应器在预定温度下恒温30 min。金属片使用前以P1200砂纸打磨至光亮,用蒸馏水冲干净,再用乙醇棉球擦拭去油污,再次用水冲干净,并用滤纸吸干水分后立即使用。将旋转圆盘调到预定转速后浸入溶液中。按一定时间间隔取样,每次取出溶液2 mL,每个实验取样6次。
样品溶液用少量王水破坏氰根,赶硝,转入25 mL容量瓶中配制成10%(体积分数)盐酸溶液待测。
铜和金样品的氰化溶解速率用分光光度法测定,铜的标准曲线相关系数为0.999 6,金的标准曲线相关系数为0.999 9。银样品的氰化溶解速率用原子吸收光谱直接测定。
氰化后的金片用蒸馏水冲洗,并在空气中自然阴干后,立即用飞利浦 XL30 ESEM-TMP扫描电镜观测其表面形貌。
2 结果
2.1 相同条件下金、银、铜氰化溶解速率的比较
在30 ℃、600 r/min转速下,金、银、铜金属片在5.00 g/L氰化钠溶液中溶解的金属浓度数据列入表1。
表1 金、银、铜氰化溶解过程中溶液金属浓度随时间的变化
Table 1 Change of concentration of gold, silver, and copper with reaction time in cyanidation dissolution process

将表1中数据换算为单位面积上金属溶解量(mol/cm2)后对时间作图得图1。
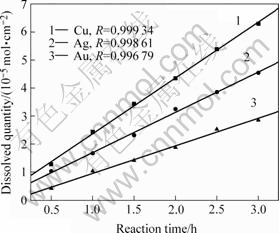
图1 在相同氰化条件下金、银、铜的溶解量与时间的关系
Fig.1 Relation of dissolved quantities of gold, silver, and copper in same cyanide solutions with reaction time
从图1中3条回归线的斜率得到金、银、铜在30 ℃ 氰化溶解的表观速率常数分别为:0.009 8、 0.014 4和0.019 9 mmol·cm-2·h-1。从图1可以明显看出,溶解速率从小至大的顺序为:Au<Ag<Cu。
2.2 硫离子对金、银、铜氰化溶解速率的影响
在与前面实验相同的条件下,氰化前向溶液中加入少量Na2S溶液,使氰化钠溶液含有5×10-6、10×10-6的硫离子,然后再用相同的方法定时取样,测定金属浓度,将所得数据列入表2并计算出溶解速率后制成图2。
表2 氰化液中含微量硫离子时金属溶解量随时间的变化
Table 2 Changes of dissolved quantities of gold, silver and copper with reaction time with Na2S added into cyanide solutions
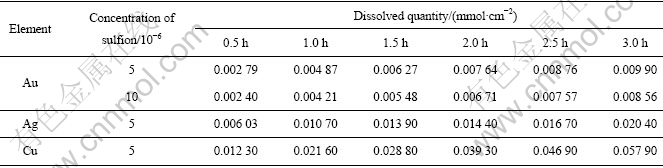
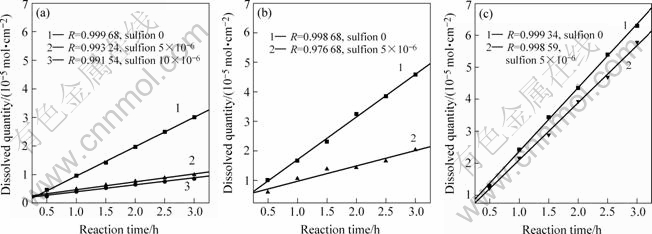
图2 微量硫离子对金、银、铜氰化溶解速率的影响
Fig.2 Influence of micro-sulfion on cyanidation dissolution rates of gold, silver and copper: (a) Au; (b) Ag; (c) Cu
比较图2 (a)~(c)可看出,5×10-6的硫离子对金的氰化溶解速率影响最大,对银的影响次之,对铜的影响最小。对金而言,硫离子浓度从5×10-6增加到10×10-6后,其影响已增加不大。
2.3 扫描电镜及能谱仪探测金、银、铜表面形态及吸附物
将表1中氰化反应前和反应后的金、银、铜片用蒸馏水冲洗,并在空气中阴干,用扫描电镜观察其形貌,如图3(a)~(d)所示。
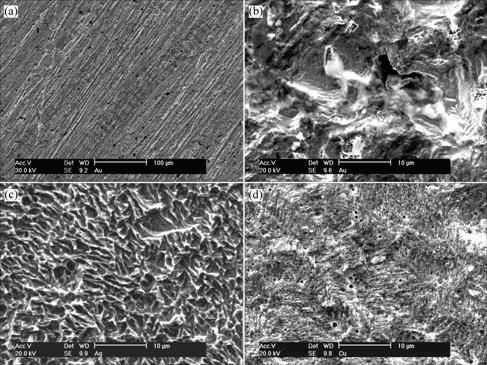
图3 氰化前及氰化3 h后的金、银、铜片形貌
Fig.3 TEM images of gold, silver and copper surface before and after cyanidation process: (a) Au, before cyanidation; (b) Au, after cyanidation; (c) Ag, after cyanidation; (d) Cu, after cyanidation
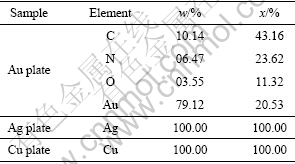
表3 氰化后金、银、铜片的表面能谱数据
Table 3 EDX data of gold, silver and copper surfaces after cyanidation process
从图3可以看出,在氰化前金片上有明显的磨 痕,银、铜片上也有相似的磨痕(图略),这些擦痕是砂纸打磨造成的。比较氰化后金、银、铜片的形貌可以看出,反应速度最慢的金片在氰化后出现尺寸较大的无规则坑状形貌,而反应速度最快的铜片在氰化后金属表面的形貌比金、银要均匀平整得多。3种金属氰化后表面的粗糙顺序为:Au>Ag>Cu,与溶解速度顺序Au<Ag<Cu相反。
由氰化后的金、银、铜表面能谱计算出样品表面元素含量,发现只在氰化后的金表面存在着用蒸馏水冲洗不掉的CN及O2。其原因可能是金对吸附物种的吸附力强,已经从物理吸附转化为化学吸附;也可能是氰化过程中银、铜表面比较平整,吸附物种容易被蒸馏水冲洗掉。
3 讨论
本研究采用600 r/min的圆盘转速,是为了使氰化溶解反应离开扩散控制区,确保在化学反应控制区进行。所获得的IB族元素的氰化表观速率常数的大小顺序为Au<Ag<Cu,这个顺序恰恰是它们化学活泼性的顺序。然而要从现有热力学、动力学以及反应机理的研究结果来解释这个顺序的理论意义却很不容易。
3.1 前人对热力学和动力学研究结果的概况
通过对金、银氰化溶解过程的热力学研究。前人提出的化学反应式有:

由于采用的热力学数据不同,文献中按式(1)~(3)计算的平衡常数K及吉布斯自由能变化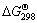 数值也不相同,有些数据甚至差别很大。虽然式(2)的K值很大,
数值也不相同,有些数据甚至差别很大。虽然式(2)的K值很大,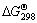 值更负,但文献作者都指出由于动力学的原因,金的氰化反应历程相当准确地符合反应式(1)。文献[13]还指明式(1)占总反应的85%,式(2)仅占总反应中的15%。
值更负,但文献作者都指出由于动力学的原因,金的氰化反应历程相当准确地符合反应式(1)。文献[13]还指明式(1)占总反应的85%,式(2)仅占总反应中的15%。
式(3)出现在文献[1]中,是卡柯夫斯基的研究结果,按式(3)计算的K值为2.3×1033,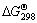 为-190.443 kJ。文献中没有说明金氰化按式(3)进行的依据或在总反应中的权重。
为-190.443 kJ。文献中没有说明金氰化按式(3)进行的依据或在总反应中的权重。
对于银的氰化溶解反应,文献中认为是与金相同的式(4)和(5):
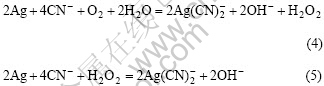
文献[3]和[13]给出上两式的K值和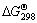 值均相同。对于式(4),K值为3×105,
值均相同。对于式(4),K值为3×105,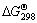 为-30.9 kJ;对于式(5),K值为5×1042,
为-30.9 kJ;对于式(5),K值为5×1042,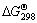 为-243 kJ。与金氰化相比,银氰化的式(4)、(5)在总反应中所占的比例相近,也就是说银的氰化反应可以用式(4)和(5)的加合式来描述。
为-243 kJ。与金氰化相比,银氰化的式(4)、(5)在总反应中所占的比例相近,也就是说银的氰化反应可以用式(4)和(5)的加合式来描述。
对于铜的氰化反应,文献[3]认为“金属铜在充气的氰化溶液中,溶解情况与贵金属相似”,反应式为:

此外,如果氰化物溶液中w(NaCN)>0.1%(质量分数)时,大部分溶解的铜将以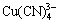 配离子存在。对于式(6)和(7),未见到热力学计算的K值和
配离子存在。对于式(6)和(7),未见到热力学计算的K值和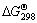 值。
值。
以上情况表明:金、银、铜氰化反应的化学计量式以及在总反应中的权重并不相同;热力学计算出的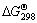 值的大小并不支配反应式在总反应中的权重;现有的热力学数据不能排列出3种金属氰化反应推动力的大小顺序。
值的大小并不支配反应式在总反应中的权重;现有的热力学数据不能排列出3种金属氰化反应推动力的大小顺序。
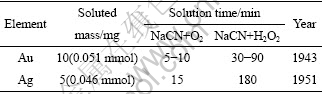
对于动力学研究,文献[3]总结了旋转圆盘法研究金、银的氰化溶解速率的一些结果,指出当圆盘转速不高(≤150 r/min)时,理论上反应在扩散控制区进行,而且“在同样条件下金和银应以同一速度溶解”。而实验测出的溶解速率数据是银略低于金,该作者认为“对此问题的最终答案尚未找到”。但是,文献[1,5]都引用了Habashi在1943年和1951年分别测定金、银氰化溶解速度的实验结果(见表4)。由表4可以看出金的溶解速度明显大于银。这些结果与本研究在w(NaCN)=0.5%、圆盘转速600 r/min及30 ℃下测得的金、银溶解速度顺序相反。
表4 金银的氰化溶解速度
Table 4 Dissolution rate of gold and silver
3.2 电化学反应机理
近年来对金氰化溶解机理的深入研究认为,溶解速率受控于金表面上发生的电化学反应。其阳极反应过程为
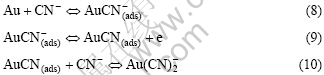
式(8)~(10)中的角标(ads)表示物种为吸附态。决定金溶解速度的是最后反应式(9)和(10)。在阳极区(+0.39~+0.64)时,最后一步式(10)占优势;在中间电 势区(-0.26~+0.39)时,则中间步骤式(9)占优势[15]。Wadsworth等[8]对于上述反应的机理还描绘出直观的图示(图4),并指出此模型预示着金的溶解行为是氰化物浓度和氧浓度的函数。Kunimastsu等[16]用Fourier-Transform红外反射光谱研究指出,线状吸附的氰离子是金表面上突出的物种。Sawaguchi等[17]使用STM、LEED及Auger电子光谱等表面表征技术研究也指出,在金表面上形成了一层AuCN。这些结果与我们用电子能谱观察到的结果一致。
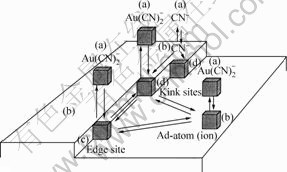
图4 Au表面发生的各种反应途径[8]
Fig.4 Gold surface illustrating various reaction paths[8]
但是,按式(8)~(10)以及图5,仍然无法解释在本研究条件下测出的氰化溶解速率是Cu>Ag>Au。
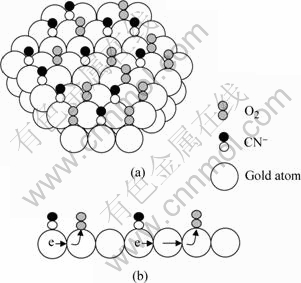
图5 CN-离子和O2分子在金表面被吸附的示意图和金表面电子转移的示意图
Fig.5 Illustration of adsorption of CN- and O2 on gold surface(a) and illustration of transport of electrons among gold atoms(b)
3.3 本文观点及实验结果解释
本文作者认为氰化溶金的表面反应应该同时考虑氧的吸附。图5(a)所示为CN-离子和水中溶解的O2分子在金表面被吸附的情况。图5(b)所示为吸附了CN- 的金原子把电子转移给吸附了O2的金原子的情况。由于氧分子可能会横着同时被两个金原子吸 附,实际情况可能会复杂一些。本文作者认为在化学反应控制的范围条件下进行氰化溶解时,金原子间电子的这种转移,以及电子离开金原子向氧分子的转移都是表面反应中的速率决定步骤,即阳极反应中的式(9)和析出电子的阴极反应都是速率决定步骤。
由于金属失去电子是氰化反应的速率决定步 骤,那么铜、银、金的氰化溶解速度将取决于失去电子的难易程度,也就是符合本研究的实验结果——铜的氰化溶解速率最快,银次之,金最慢。本文作者提出的同时吸附CN-及O2的模型还可满意地解释前人研究动力学所证实的结论——当溶液中氧的浓度高时,金的溶解速率随氰离子浓度的增大而增大;当溶液中氰离子浓度高时,反应速率则随氧浓度的增大而增大。
此外,图5所示模型也符合早期的许多研究结果。如:1) [CN-]/[O2]有一个最佳的临界值,相当于金表面上吸附CN-的原子数与吸附O2的原子数有一个最有利于反应进行的比值;2) 在扩散控制区反应时,与氧能否高速供给到金表面有关,但过高的氧浓度反而会降低氧化速率,提高了反应的活化能等。
3.4 硫离子降低氰化反应速率的原因及影响顺序的解释
在氧的存在下,硫离子能使CN-转化为CNS-,而且更重要的是它强烈的吸附在金的表面,使金表面钝化而抑制了金的氰化溶解速率。这种现象可以从Au对硫的亲合力大于对CN-和O2,S2-可以取代被吸附的CN-和O2,从而导致金氰化溶解速率的显著降低来解释。
对于银和铜吸附CN-、O2、S2-的能力,也可从有关化合物的热力学数据(见表5)来分析。
表5 银和铜有关化合物的生成焓(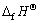 )数据1)
)数据1)
Table 5 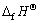 value of Ag and Cu compounds
value of Ag and Cu compounds

从表5中的数据可见,虽然AgCN和CuCN生成焓都为正值,但后者更易生成。Ag2O和Ag2S的 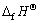 值相近,S2-可与O2发生竞争吸附。Cu2O和Cu2S相比,铜将优先吸附O2。
值相近,S2-可与O2发生竞争吸附。Cu2O和Cu2S相比,铜将优先吸附O2。
S2-难以取代铜片上吸附的O2,导致铜的氰化溶解速率基本上不受S2-的影响。此外也可以从Cu2S可以溶解于NaCN溶液来说明S2-的存在不影响铜的氰化反应。以上推断可以较满意地解释本文研究实验结果,但由于3种金属氰化反应的复杂性,对于硫离子的影响机理还有必要进行深入研究。
3.5 氰化后金表面形貌的解释
金、银、铜片在氰化反应前的形貌相似,纹络都属抛光的划痕。金在氰化3 h后形成分散的坑状腐蚀形貌,说明金表面开始发生反应后的位置周边会形成一些表面的活性位点,表面反应不断在这些活性点上进行,进而形成较大的坑穴。这种现象与Wadsworth提出的金表面反应模型(图4)相符合。同时从金氰化后表面元素能谱分析的结果也可以看出,金表面反应中存在对CN-和O2的吸附。铜片上的氰化反应速度很快,氰化反应后的表面形貌呈较均匀的腐蚀状。银的反应速度居中,坑穴较小,较均匀。
4 结论
1) 用旋转圆盘法,在氰化钠浓度为5.00 g/L (质量分数为0.5%)、圆盘转速600 r/min、温度30 ℃时,获得金、银、铜的氰化表观速率常数分别为0.009 8、0.014 4和0.019 9 mmol·cm-2·h-1,表明氰化溶解的速度顺序是:Au<Ag<Cu。
2) 当氰化液中含有5×10-6的硫离子时,金、银、铜的氰化都受到抑制,其影响程度顺序为:Au>Ag>Cu。
3) 提出了CN-和O2在金属表面同时吸附的模型,认为在化学反应控制区条件下,金属原子给出电子,如吸附态的AuCN- 转化为吸附态的AuCN+e,以及O2从金属原子夺取电子是速率控制的步骤,可以较满意的解释Au<Ag<Cu的溶解速率顺序。氰化后金表面的能谱分析符合本文作者提出的模型。
4) 硫离子抑制氰化溶解速度的顺序可以从生成焓推断的金亲硫强于亲氧,铜亲氧强于亲硫,银居中。因而硫离子对金的氰化影响最大,对铜影响最小而获得解释。
REFERENCES
[1] 黄礼煌. 金银提取技术[M]. 北京: 冶金工业出版社, 2001: 133-147.
HUANG Li-huang. Extraction Technique of Gold and Silver[M]. Beijing: Metallurgical Industry Press, 2001: 133-147.
[2] 赵怀志, 宁远涛. 金[M]. 长沙: 中南大学出版社, 2003: 50.
ZHAO Huai-zhi, NING Yuan-tao. Gold[M]. Changsha: Central South University Press, 2003: 50.
[3] Masleniski N H, 等. 贵金属冶金学[M]. 北京: 原子能出版社, 1992: 72-106.
Masleniski N H, et al. Precious Metal Metallurgy[M]. Beijing: Atomic Energy Press, 1992: 79-106.
[4] Kondos P D, Morrison P M. Process optimization studies in gold cyanidation[J]. Hydrometallurgy, 1995, 39: 235-250.
[5] 孙 戬. 金银冶金[M]. 北京: 冶金工业出版社, 1986: 77.
SUN Jian. Metallurgy of Gold and Silver[M]. Beijing: Metallurgical Industry Press, 1986: 77.
[6] Yannopoulos J C. 金的提取冶金[M]. 田广荣, 李关芳, 蒋鹤麟, 译. 昆明: 中国有色金属工业贵金属信息网, 1992: 107.
Yannopoulos J C. Extractive Metallurgy of Gold [M]. TIAN Guang-rong, LI Guan-fang, JIANG He-ling, transl. Kunming: Information-net of Chinese Nonferrous Metals Industry of Noble Metals, 1992, 107.
[7] Wadsworth M E. Surface processes in silver and gold cyanidation[J]. Int J Miner Process, 2000, 58: 351-368.
[8] Wadsworth M E, Zhu X, Thompson J S, Pereira C J. Gold dissolution and activation in cyanide solution: kinetics and mechanism[J]. Hydrometallurgy, 2000, 57: 1-11.
[9] 黎鼎鑫, 王永录. 贵金属提取与精炼[M]. 长沙: 中南大学出版社, 2003: 153.
LI Ding-xin, WANG Yong-lu. The Extraction and Refinery of Precious Metals[M]. Changsha: Central South University Press, 2003: 153.
[10] 涂光炽,刘秉光,王秀璋. 金矿——人类最早认识和利用的矿产[M]. 北京: 清华大学出版社及暨南大学出版社, 2002: 102.
TU Guang-chi, LIU Bing-guang, WANG Xiu-zhang. Gold Ores—The Primal Known and Utilized Ores[M]. Beijing: Tsinghua University Press and Jinan University Press, 2002: 102.
[11] 蔡 玲, 孙长泉. 伴生金银综合回收[M]. 北京: 冶金工业出版社, 1999: 30.
CAI Ling, SUN Chang-quan. Recovery of Gold and Silver from Their Symbiotic Minerals[M]. Beijing: Metallurgical Industry Press, 1999: 30.
[12] 黄初登. 金矿床成因、勘探与贵金属回收[M]. 北京: 冶金工业出版社, 2000: 40-43.
HAUNG Chu-deng. Formation and Exploration of Gold Ores and Recovery of Precious Metals[M]. Beijing: Metallurgical Industry Press, 2000: 40-43.
[13] 卢宜源, 宾万达. 贵金属冶金学[M]. 长沙: 中南大学出版社, 2004: 54-80.
LU Yi-yuan, BIN Wan-da. Precious Metal Metallurgy[M]. Changsha: Central South University Press, 2004: 54-80.
[14] Adamson R J. Gold Metallurgy in South Africa[M]. 1973: 292.
[15] 《黄金生产工艺指南》编委会. 黄金生产工艺指南[M]. 北京: 地质出版社, 2000: 131.
Editors of Guide for Gold Extraction Technique. Gold Extraction Technique[M]. Beijing: The Geological Press, 2000: 131.
[16] Kunimatsu K, Seki H, Golden W G, Gordon J G Ⅱ, Philpott M R. A Study of the Gold/Cyanide Solution Interphase by in-situ Polarization Modulated FT IRRAS[J]. Langmuir, 1988, 4: 337-341.
[17] Sawaguchi T, Yamada T, Okinaka Y, Itaya K. Electrochemical scanning tunneling microscopy and ultrahigh-vacuum investigation of gold cyanide adlayers on Au(III) formed in aqueous solution[J]. J Phys Chem, 1995, 99: 14149-14155.
[18] Dean J A. 兰氏化学手册[M](第15版). 魏俊发, 等译. 北京: 科学出版社, 2003.
Dean J A.. Lange’s Handbook of Chemistry (Version 15) [M]. WEI Jun-fa, et al, transl. Beijing: Science Press, 2003.
基金项目:国家自然科学基金资助项目(50374060)
收稿日期:2006-05-12;修订日期:2006-11-12
通讯作者:陈 景,教授,中国工程院院士;电话:0871-5032180;E-mail: chjing@ynu.edu.cn
(编辑 何学锋)