文章编号:1004-0609(2008)02-0356-05
碳酸钠分解白钨矿的热力学分析
赵中伟,曹才放,李洪桂
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:通过热力学计算,绘制了25 ℃下白钨矿在总碳浓度为1 mol/L Na2CO3溶液中各主要溶解组分的lg c—pH图,以及白钨矿在不同总碳浓度下Na2CO3溶液中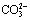 和
和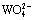 的lg c—pH图。利用这些热力学平衡图对Na2CO3分解白钨矿的工艺条件进行了分析,认为溶液中
的lg c—pH图。利用这些热力学平衡图对Na2CO3分解白钨矿的工艺条件进行了分析,认为溶液中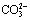 浓度直接影响白钨矿分解,而当碱度较低时,Na2CO3将水解生成
浓度直接影响白钨矿分解,而当碱度较低时,Na2CO3将水解生成 ,从而降低
,从而降低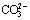 浓度,进而降低白钨矿的分解率;故在工业实践中,为使钨最大限度地转入溶液,除了保证足够的Na2CO3用量,还必需添加NaOH以维持一定碱度。利用热力学分析所得结论,重新解释了前人的实验现象,认为以过多的NaOH代替Na2CO3造成钨浸出率回落的原因在于,溶液中
浓度,进而降低白钨矿的分解率;故在工业实践中,为使钨最大限度地转入溶液,除了保证足够的Na2CO3用量,还必需添加NaOH以维持一定碱度。利用热力学分析所得结论,重新解释了前人的实验现象,认为以过多的NaOH代替Na2CO3造成钨浸出率回落的原因在于,溶液中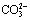 浓度的较大幅度下降,而不是钨矿中脉石分解所引起的Na2CO3消耗。
浓度的较大幅度下降,而不是钨矿中脉石分解所引起的Na2CO3消耗。
关键词:白钨矿;碳酸钠分解;热力学;浓度对数图
中图分类号:TF 111.31 文献标识码:A
Thermodynamics on soda decomposition of scheelite
ZHAO Zhong-wei, CAO Cai-fang, LI Hong-gui
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083,China)
Abstract: The lg c—pH diagrams at 25 ℃ for containing ions when the total carbon concentration being 1 mol/L are established according to thermodynamic calculation. The lg c—pH diagrams for 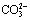 and
and 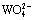 at different total carbon concentrations are also established. Using these diagrams, thermodynamic analysis is carried out to show the effect of technical conditions on soda decomposition of scheelite. It appears that the concentration of
at different total carbon concentrations are also established. Using these diagrams, thermodynamic analysis is carried out to show the effect of technical conditions on soda decomposition of scheelite. It appears that the concentration of 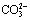 has direct effect on soda decomposition of scheelite. When the alkalinity is low,
has direct effect on soda decomposition of scheelite. When the alkalinity is low, 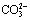 will hydrolyze to
will hydrolyze to 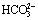 , accordingly the decomposition rate of scheelite becomes lower because of the decrease of
, accordingly the decomposition rate of scheelite becomes lower because of the decrease of 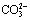 . So in order to get higher leaching rate in industry practice, it is necessary to use enough amount of soda to serve as digestion reagent and, certain amount of NaOH to maintain high enough alkalinity. A re-explanation of experimental phenomenon reported in literature is derived, which shows that the reason causing the decrease of tungsten extraction when using overhigh degree of substitution of NaOH for Na2CO3 lies in the sharp decrease of the
. So in order to get higher leaching rate in industry practice, it is necessary to use enough amount of soda to serve as digestion reagent and, certain amount of NaOH to maintain high enough alkalinity. A re-explanation of experimental phenomenon reported in literature is derived, which shows that the reason causing the decrease of tungsten extraction when using overhigh degree of substitution of NaOH for Na2CO3 lies in the sharp decrease of the 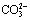 concentration, not the increasing attack of gangue constituents, which would consume Na2CO3 that otherwise would have been available to digest scheelite.
concentration, not the increasing attack of gangue constituents, which would consume Na2CO3 that otherwise would have been available to digest scheelite.
Key words: scheelite; soda decomposition; thermodynamics; concentration logarithm diagram
碳酸钠压煮法适合于处理白钨精矿和中间产品,它是HAMILTON[1]提出的。该法基于如下反应:
CaWO4(s)+Na2CO3(aq)=Na2WO4(aq)+CaCO3(s)
白钨矿在高温高压下与碳酸钠溶液反应生成可溶性的钨酸钠与不溶的碳酸钙,从而将矿物中的钨浸出至溶液。该方法自提出以来,很多学者对其热力学和动力学进行了研究[2-7]。QUENEAU等[8-12]针对该法处理白钨矿及黑白钨混合矿申请了多项专利,李洪桂等利用机械活化强化了该分解过程[13-15]。在热力学研究方面,OSSEO-ASARE[2]绘制了25 ℃下该体系的ε—pH图,如图1所示。同时,国内也有学者在OSSEO- ASARE的基础上进一步绘制了更高温度或浓度的ε—pH图[3-4]。然而,由反应式可知,白钨矿的碳酸钠分解过程是一个复分解反应,而不是氧化还原反应,反应过程没有涉及电子得失,因而ε—pH图纵坐标提供的电势信息对浸出过程只具有一定的参考价值,能粗略地知道碳酸钠分解白钨矿需维持体系pH值高于一定数值(约为7~8),如图1所示。而采用溶解组分lg c—pH图,则可以用纵坐标清楚地表示溶液中各组分的平衡浓度随pH值变化的趋势,更适合于分析白钨矿的浸出过程[16-18]。本文作者利用文献[2]中的数据,就白钨矿的碳酸钠分解绘制了溶解组分的lg c—pH图,并对分解过程的热力学进行了分析。
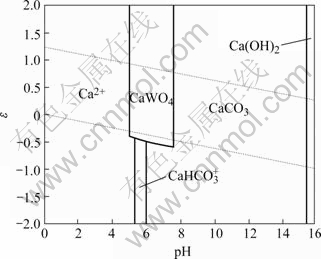
图1 Ca-W-C-H2O体系的ε—pH图
Fig.1 ε—pH diagram for Ca-W-C-H2O system ([W]=[Ca]=10-3 mol/L, [C]=1mol/L, 25 ℃)
1 溶解组分lg c—pH图的绘制
溶液中存在如表1所列的平衡关系式,所有数据均引自文献[2]。在缺少有关离子的活度系数的情况下,下面计算均以浓度代替活度。
由这些平衡关系式可以看出,溶液中游离Ca、W和C的存在形态有:Ca2+、CaOH-,CaHCO3+、CaCO3(aq)、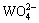 、H2WO4(aq)、
、H2WO4(aq)、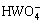 、
、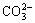 、H2CO3和
、H2CO3和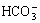 ,由表1中的平衡关系式(5)~(11)可知,它们的平衡浓度满足以下方程:
,由表1中的平衡关系式(5)~(11)可知,它们的平衡浓度满足以下方程:

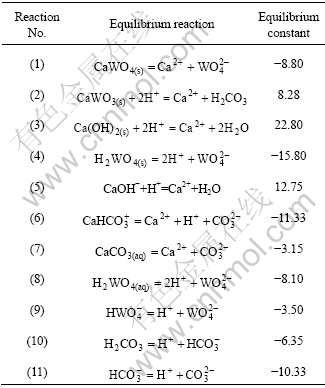
表1 Ca-W-C-H2O体系的平衡反应及其平衡常数
Table 1 Equilibrium reactions and constants for Ca-W-C-H2O system (25 ℃)
各含碳溶解组分浓度总和等于总碳浓度:

由平衡关系式可以看出,在一定条件下溶液中会生成各种沉淀,它们的溶解平衡应分别加以考虑。当体系中只有CaWO4(s)而无别的沉淀生成,则溶液中各游离组分满足方程(1)~(10);在pH值较低的时候,会有H2WO4(s)生成,这时溶液中各游离组分满足方程(1)~(9)、(11);当溶液pH值升高时,会有CaCO3(s)生成,各游离组分满足方程(1)~(9)、(12);当pH较高时,CaCO3(s)会进一步转化为Ca(OH)2(s),各游离组分满足方程(1)~(9)、(13)。
当总碳浓度为1 mol/L时,求得生成CaCO3(s)的边界pH值为6.9,生成H2WO4(s)的边界pH值为5.8,生成Ca(OH)2(s)的边界pH值为15.6。根据以上计算和平衡关系,可以绘制25 ℃时Ca-W-C-H2O体系溶液组分的lg c—pH图,如图2所示。
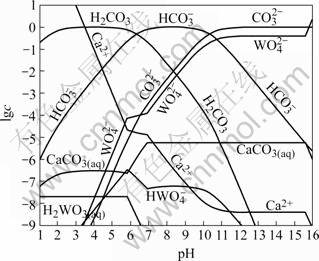
图2 Ca-W-C-H2O体系溶解组分的lg c—pH图
Fig.2 lg c—pH diagram for Ca-W-C-H2O system (25 ℃, Total carbon concentration 1 mol/L)
这种方法亦可计算出总碳浓度分别为0、0.001、0.1和2 mol/L时25 ℃下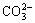 和
和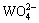 的lg c—pH图,如图3所示。
的lg c—pH图,如图3所示。
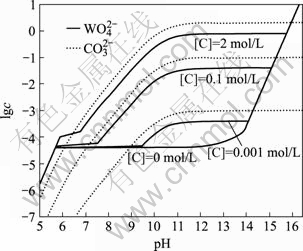
图3 不同碳酸钠浓度时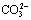 和
和 的lg c—pH图
的lg c—pH图
Fig.3 lg c—pH diagram for 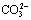 and
and  at different soda concentrations (25 ℃)
at different soda concentrations (25 ℃)
2 碳酸钠分解白钨矿的条件分析
由图2可知,碳酸钠是分解白钨矿的有效试剂。在图2给定的条件下,CaWO4的稳定区域很小,仅在5.8<pH<6.9范围内稳定,pH值高于或低于这个范围,白钨矿开始分解。
碳酸钠分解白钨矿生成产物为CaCO3,因此溶液中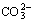 浓度直接影响白钨矿分解。如图2所示,在pH<6.4时,
浓度直接影响白钨矿分解。如图2所示,在pH<6.4时,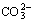 会结合H+生成H2CO3,进而分解释放CO2,使碳酸钠分解,因而此时讨论碳酸钠分解白钨矿实际是没有意义的。
会结合H+生成H2CO3,进而分解释放CO2,使碳酸钠分解,因而此时讨论碳酸钠分解白钨矿实际是没有意义的。
在6.4<pH<10.4时,碳酸钠水解主要以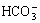 形式存在。由于
形式存在。由于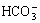 不能与Ca2+形成难溶化合物,故此区域碳酸钠未能被充分利用。然而,随着pH值升高,
不能与Ca2+形成难溶化合物,故此区域碳酸钠未能被充分利用。然而,随着pH值升高,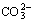 浓度升高,其分解白钨矿所生成的CaCO3沉淀逐渐能稳定存在。
浓度升高,其分解白钨矿所生成的CaCO3沉淀逐渐能稳定存在。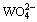 浓度亦随着
浓度亦随着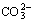 浓度急剧升高而升高,且变化趋势一致;当pH>10.4时,
浓度急剧升高而升高,且变化趋势一致;当pH>10.4时,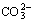 成为碳酸钠在溶液中的主要存在形态,此区域中,
成为碳酸钠在溶液中的主要存在形态,此区域中,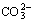 平衡浓度曲线逐渐趋于一“平台”,
平衡浓度曲线逐渐趋于一“平台”, 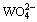 平衡浓度在10.4<pH<15.6时亦有一“平台”存在,这时pH值对
平衡浓度在10.4<pH<15.6时亦有一“平台”存在,这时pH值对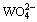 浓度没有太大的影响。因此,要保证白钨矿被碳酸钠有效分解,要维持一定碱度抑止碳酸钠水解。这一点可以解释QUENEAU[8]的研究结果,他发现在其它条件相同时,pH=12.0时的分解效果要优于pH=8.3。当然,这只是从热力学角度而言。
浓度没有太大的影响。因此,要保证白钨矿被碳酸钠有效分解,要维持一定碱度抑止碳酸钠水解。这一点可以解释QUENEAU[8]的研究结果,他发现在其它条件相同时,pH=12.0时的分解效果要优于pH=8.3。当然,这只是从热力学角度而言。
当pH>15.6时,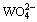 平衡浓度开始增加,这时CaCO3将分解成为Ca(OH)2,分解过程转变为碱分解过程,碳酸钠实际上已不起作用。对于低品位矿,由于伴生有CaCO3,用NaOH分解时,使得NaOH用量在本来就已相当高的基础上进一步升高,因而用NaOH分解含钙高的钨矿是不适宜的。
平衡浓度开始增加,这时CaCO3将分解成为Ca(OH)2,分解过程转变为碱分解过程,碳酸钠实际上已不起作用。对于低品位矿,由于伴生有CaCO3,用NaOH分解时,使得NaOH用量在本来就已相当高的基础上进一步升高,因而用NaOH分解含钙高的钨矿是不适宜的。
由图3可知,增加碳酸钠用量可较大幅度地改善分解。在碳酸钠分解白钨矿时,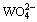 和
和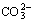 浓度变化曲线的变化趋势是一致的。这说明:为了使钨最大限度地转入溶液,必需保证足够碳酸钠用量,并维持一定碱度抑止碳酸钠水解。这与人们的实验研究也是一致的。
浓度变化曲线的变化趋势是一致的。这说明:为了使钨最大限度地转入溶液,必需保证足够碳酸钠用量,并维持一定碱度抑止碳酸钠水解。这与人们的实验研究也是一致的。
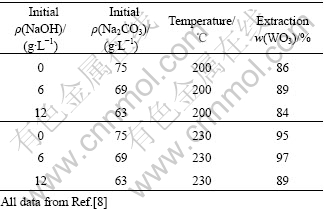
文献[8]曾报道以NaOH代替部分Na2CO3会有益于分解,如表2所列。这说明在添加NaOH的同时,Na2CO3用量可望有一定程度降低。表2还说明,以较多的NaOH代替Na2CO3时,分解率反而下降。原文作者认为是由于增加了脉石的分解,消耗了本来用于分解钨矿的试剂,但本文作者对此有不同的解释。由图2可知,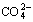 浓度在高pH值下有一“平台”。在此之前pH值降低,
浓度在高pH值下有一“平台”。在此之前pH值降低,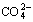 浓度迅速降低。如果以NaOH代替部分Na2CO3,会产生两种相反影响:1) 由于Na2CO3用量减少,分解率有降低趋势;2) 由于pH值的增加,有利于抑止碳酸钠水解,使之以
浓度迅速降低。如果以NaOH代替部分Na2CO3,会产生两种相反影响:1) 由于Na2CO3用量减少,分解率有降低趋势;2) 由于pH值的增加,有利于抑止碳酸钠水解,使之以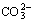 存在于溶液,于是
存在于溶液,于是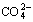 浓度又有增加趋势。由于分解过程中的副反应,不可避免地会使pH值降低。在这种情况下,就相当于体系位于平台前的陡坡上。此时,添加NaOH的影响最大,而Na2CO3用量的轻微改变对体系影响相对较小。结果以NaOH代替少部分Na2CO3使分解效果改善了。若以NaOH代替更多的碳酸钠,虽可使pH值进一步增加,但由图3可知,pH超过一定值后,再增加NaOH无明显影响,而Na2CO3较大幅度减少的不利影响相对突出,结果使分解率反而回落。
浓度又有增加趋势。由于分解过程中的副反应,不可避免地会使pH值降低。在这种情况下,就相当于体系位于平台前的陡坡上。此时,添加NaOH的影响最大,而Na2CO3用量的轻微改变对体系影响相对较小。结果以NaOH代替少部分Na2CO3使分解效果改善了。若以NaOH代替更多的碳酸钠,虽可使pH值进一步增加,但由图3可知,pH超过一定值后,再增加NaOH无明显影响,而Na2CO3较大幅度减少的不利影响相对突出,结果使分解率反而回落。
表2 以NaOH代替Na2CO3对分解的影响
Table 2 Effects of NaOH replacing part of Na2CO3 on decomposition
3 结论
1) 运用现有热力学数据,通过计算,绘制了25 ℃下白钨矿在总碳浓度为1 mol/L的Na2CO3溶液中溶解组分的lg c—pH图以及不同Na2CO3浓度时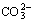 和
和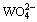 的lg c—pH图。
的lg c—pH图。
2) 运用热力学平衡图,分析了Na2CO3分解白钨矿的热力学条件,认为溶液中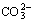 浓度直接影响白钨矿分解。当碱度较低时,Na2CO3将水解生成
浓度直接影响白钨矿分解。当碱度较低时,Na2CO3将水解生成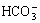 ,从而降低
,从而降低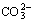 浓度,进而降低白钨矿的分解率。为了使钨最大限度地转入溶液,必需保证足够Na2CO3用量,并维持一定碱度以抑止Na2CO3水解。
浓度,进而降低白钨矿的分解率。为了使钨最大限度地转入溶液,必需保证足够Na2CO3用量,并维持一定碱度以抑止Na2CO3水解。
3) 利用热力学平衡图,重新解释了前人的实验现象,认为以过多的NaOH代替Na2CO3造成钨浸出率回落的原因在于溶液中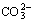 浓度较大幅度地下降,而不是钨矿中脉石分解所引起的Na2CO3消耗。
浓度较大幅度地下降,而不是钨矿中脉石分解所引起的Na2CO3消耗。
REFERENCES
[1] HAMILTON E M. Extraction of metals from their ores[P]. US1399554, 1921-12-06.
[2] OSSEO-ASARE K. Solution chemistry of tungsten leaching systems[J]. Metallurgical Transaction B, 1982, 13: 555-564.
[3] 李自强, 何良惠. W-H2O、Ca-W-H2O、Mn-W-H2O、Fe-W-H2O及Ca-W-CO3-H2O系电位—pH图在湿法分解钨矿石中的应用[J]. 四川冶金, 1984(4): 62-67.
LI Zi-qiang, HE Liang-hui. Application of Eh-pH diagrams of the W-H2O, Ca-W-H2O, Mn-W-H2O, Fe-W-H2O and Ca-W-CO3-H2O systems in digestion of tungsten ore[J]. Sichuan Metallurgy, 1984(4): 62-67.
[4] 李正山, 何良惠, 郑昌琼. 钨矿及钨锡中矿碳酸钠加压浸出热力学分析[J]. 稀有金属, 1992(1): 1-6.
LI Zheng-shan, HE Liang-hui, ZHENG Chang-qiong. Thermodynamic analysis of autoclave soda digestion of tungsten ore and tungsten-tin middle concentrate[J]. Chinese Journal of Rare Metals, 1992(1): 1-6.
[5] MARTINS J P. Kinetics of soda ash leaching of low-grade scheelite concentrates[J]. Hydrometallurgy, 1996, 42(2): 221-236.
[6] MARTINS J P, MARTINS F. Soda ash leaching of scheelite concentrates: The effect of high concentration of sodium carbonate[J]. Hydrometallurgy, 1997, 46(1): 191-203.
[7] CHO E H. Kinetics of sodium carbonate leaching of scheelite[J]. Journal of Metals, 1988, 40(7): 32-34.
[8] QUENEAU P B, HUGGINS D K, BECKSTEAD L W. Soda ash digestion of scheelite concentrates[C]//SOHN H Y, CARLSON O N, SMITH J T. Extractive Metallurgy of Refractory Metals. New York: The Metallurgical Society of AIME, 1981: 237-267.
[9] QUENEAU P B, HUGGINS D K, BECKSTEAD L W. Autoclave soda digestion of refractory scheelite concentrates[P]. US4320095, 1982-03-16.
[10] QUENEAU P B, HUGGINS D K, BECKSTEAD L W. Combined autoclave soda digestion of wolframite and scheelite[P]. US4320096, 1982-03-16.
[11] QUENEAU P B, BECKSTEAD L W, HUGGINS D K. Autoclave soda digestion of scheelite concentrates with feedback control[P]. US4325919, 1982-04-20.
[12] BECKSTEAD L W, HUGGINS D K, QUENEAU P B. Autoclave soda digestion of scheelite concentrates[P]. US4351808, 1982-09-28.
[13] 刘茂盛, 李洪桂, 孙培梅, 李运姣, 苏鹏抟, 赵中伟. 机械活化苏打溶液分解白钨中矿试验研究[J]. 中南工业大学学报(自然科学版), 1994, 25(3): 321-325.
LIU Mao-sheng, LI Hong-qui, SUN Pei-mei, LI Yun-jiao, SU Peng-tuan, ZHAO Zhong-wei. Study on mechanical activated decomposition with soda solution of scheelite middle concentrate[J]. Journal of Central South University of Technology(Natural Science), 1994, 25(3): 321-325.
[14] 赵中伟, 李洪桂, 刘茂盛, 孙培梅, 李运姣, 苏鹏抟. 柿竹园钨中矿处理方法选择[J]. 稀有金属与硬质合金, 1996, 24(1): 1-3.
ZHAO Zhong-wei, LI Hong-qui, LIU Mao-sheng, SUN Pei-mei, LI Yun-jiao, SU Peng-tuan. Method selection for treatment of Shizhuyuan low-grade tungsten ore[J]. Rare Metals and Cemented Carbides, 1996, 24(1): 1-3.
[15] 赵中伟. 钨冶炼理论、工艺研究[D]. 长沙: 中南工业大学, 1997.
ZHAO Zhong-wei. Study on the theories and processes of tungsten metallurgy[D]. Changsha: Central South University of Technology, 1997.
[16] 丁治英, 赵中伟. 氟盐溶液浸出白钨矿的热力学分析[J]. 稀有金属与硬质合金, 2004, 32(1): 8-10.
DING Zhi-ying, ZHAO Zhong-wei. Thermodynamic analysis of scheelite leaching by fluoride solution[J]. Rare Metals and Cemented Carbides, 2004, 32(1): 8-10.
[17] 王识博, 赵中伟, 李洪桂. 磷酸盐浸出白钨矿的热力学分析[J]. 稀有金属与硬质合金, 2005, 33(1): 1-4.
WANG Shi-bo, ZHAO Zhong-wei, LI Hong-qui. Thermodynamic analysis on phosphate decomposition of scheelite[J]. Rare Metals and Cemented Carbides, 2005, 33(1): 1-4.
[18] 史海燕, 赵中伟. 苛性钠分解黑钨矿的热力学分析[J]. 中国钨业, 2006, 21(5): 24-27.
SHI Hai-yan, ZHAO Zhong-wei. Thermodynamics analysis on caustic sodium decomposition of wolframite[J]. China Tungsten Industry, 2006, 21(5): 24-27.
基金项目:新世纪优秀人才支持计划资助项目(NCET-05-0692);国家自然科学基金资助项目(50344031)
收稿日期:2007-05-08;修订日期:2007-09-15
通讯作者:赵中伟,教授,博士;电话:0731-8830476; E-mail: zhongweizhao@hotmail.com
(编辑 何学锋)