文章编号:1004-0609(2013)06-1746-11
铝电解过程中碱金属的电化学插层及渗透迁移行为
方 钊,武小雷,俞 娟,李林波,朱 军
(西安建筑科技大学 冶金工程学院,西安 710055)
摘 要:采用改进型阴极电解膨胀率测试仪,结合电解后试样剖面元素线扫描和面扫描(SEM)的结果,研究电解过程中碱金属K、Na在TiB2-C复合阴极中的渗透迁移行为;同时采用循环伏安法研究K、Na的电极电化学行为,结果表明:电解过程中,碱金属K、Na均渗透进入了阴极内部,K在阴极中的扩散系数及所引起的最大阴极电解膨胀率分别为2.86×10-5 cm2/s和1.35%,均高于Na的,说明K的渗透力强于Na的。碱金属K、Na在TiB2-C复合阴极中表现出了相似的渗透迁移路径,碱金属K、Na首先随电解质一同渗透进入阴极的孔隙当中,随后又渗透进入粘结剂结焦碳中,随着电解的进行,最终渗透进入阴极碳质骨料中,但K、Na不会渗透进入TiB2颗粒中。渗透进入粘结剂和碳质骨料中的K、Na均会引起阴极的电解膨胀,并随阴极中碱金属浓度的饱和而达到平衡。而对碱金属K、Na电极行为的研究表明:电解过程中,K、Na会在阴极表面共同析出,但在阳极过程中,与Na相比,C-K插层化合物所表现出的氧化电势更正,C-K插层化合物的稳定性更高,插入石墨层间形成插层化合物的K很难脱出,其对阴极的破坏力较Na的更强。
关键词:铝电解;阴极;碱金属;渗透;迁移;插层
中图分类号:TF821 文献标志码:A
Electrochemical insertion and penetration and migration behavior of alkali metal in aluminum electrolysis process
FANG Zhao, WU Xiao-lei, YU Juan, LI Lin-bo, ZHU Jun
(School of Metallurgical Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, China)
Abstract: During aluminum electrolysis, the electrochemical insertions of alkali metals (potassium and sodium) as well as their migratory behavior in TiB2-C composite cathodes was investigated by means of line scanning and map scanning through SEM and self-made modified Rapoport apparatus. The electrochemical behavior of alkali metals on the electrode was also studied by cyclic voltammetry. The results suggest that, in the aluminum electrolysis process, both K and Na penetrate into the cathode, the diffusion coefficient of K in the cathode and the induced maximal electrolysis expansion are 2.86×10-5 cm2/s and 1.35%, respectively, both of which are higher than those of Na, illustrating that the penetration ability of K is stronger than that of Na. Meanwhile, the penetration and migration path of K and Na in TiB2-C composite cathode is similar: firstly, alkali metals (K and Na) penetrate into the pores of the cathode together with the electrolyte, subsequently into the binder coke, with the progress of the electrolysis, finally into the carbonaceous aggregates of the cathode, while K and Na can not penetrate into TiB2. K and Na penetrate into both binder and aggregates, leading to the expansion of TiB2-C composite cathodes, which will become constant when the content of alkali metals (K and Na) in the cathode is saturated. While the results on the electrode behavior of alkali metals K and Na indicate that, in the aluminum electrolysis process, K and Na will co-deposit on cathode, while during the anodic process, the intercalation compound of K represent more positive potential than that of Na, illustrating better stability of intercalation compound of K. K inserted into the interlayer of carbon materials is difficult to break away, the destructive force is stronger than that of Na.
Key words: aluminum electrolysis; cathode; alkali metals; penetration; migration; insertion
铝电解工业是世界各国十分关注的重要基础原材料产业,但巨大的能源消耗、资源消费和环境负荷严重制约着铝工业的发展[1-2]。基于惰性电极材料(惰性阳极和可润湿性阴极)的铝电解技术可消除温室气体CO2、致癌物质CFn以及沥青烟气的排放,实现电解槽的低能耗运行,因而成为世界铝业界和学术界的关注焦点和研究热点[3-4]。研究表明,在现行电解工艺条件下,所制备的惰性阳极(包括氧化物陶瓷阳极、金属陶瓷阳极以及金属或合金阳极等),其耐高温熔盐腐蚀性能,或其抗热震性能,还难以满足铝电解工业生产的要求,不能获取高品质原铝[5]。这些问题的出现,使得惰性阳极无法单独使用,必须与低温铝电解工艺(较为友好的服役环境)和惰性可润湿性阴极(较低的阴阳极极距)配合使用才能达到真正节能降耗的目的,也就是说,对于惰性电解系统而言,低温铝电解是不可或缺的重要组成之一[6-9]。然而,电解温度的降低势必会引起电解质当中Al2O3溶解能力的下降,造成电解过程中槽底出现Al2O3沉淀,导致电解槽无法正常稳定运行[10],因此,解决好低电解温度与Al2O3溶解性能下降之间的矛盾至关重要。
钾冰晶石(K3AlF6)熔体由于能够在保证低温电解的同时,保持较高的Al2O3溶解度和良好的运行稳定性,成为低温铝电解工艺的研究热点和关注焦点[11-13]。但钾盐的使用,会对铝电解用阴极材料产生较强的(膨胀)破坏作用。与传统电解质体系中的Na相比,K的离子势更低,电解过程中,更加容易渗透进入阴极内部,形成相应的C-K插层化合物,对阴极产生强烈的破坏作用,严重影响铝电解槽的使用寿命和正常的工业生产。GALASIN等[14]的研究表明,单一钾冰晶石作为电解质,阴极使用寿命将大幅缩短,并最终导致电解槽的早期破损。NAAS等[15]则以等量的KF替代NaF进行研究,发现,在钾盐添加量≤5%(摩尔分数)的条件下,其对半石墨质阴极的电解膨胀率几乎没有影响;但当钾盐添加量为20%(摩尔分数)时,所引起的阴极电解膨胀率是不添加KF时电解膨胀率的2倍。LI等[16]的研究也证实了上述结论,认为钾冰晶石的添加量不宜超过10%(质量分数),否则将会引起半石墨质阴极较大的电解膨胀率,也就是说,大量使用钾盐,虽对电解质熔体初晶温度的降低和氧化铝溶解性能的提升作用明显,但其对传统阴极的破坏作用也剧烈增加。可以看出,半石墨质阴极抵御含钾电解质侵蚀的能力有限,其应用于生产时,电解质中钾盐的添加量只能控制在较少的范围内,这对于电解质熔体的降温及氧化铝溶解性能的提升作用有限。为了在充分发挥钾盐优势的同时减小其对阴极的破坏作用,迫切需要开发一种具有较好抗碱金属侵蚀性能的铝电解阴极。TiB2基可润湿性阴极应运而生,成为一种很有潜力、能够抵御含钾低温电解质熔体强腐蚀性的铝电解惰性电极系统用阴极材料[17-19]。FANG等[20]在含钾低温电解质熔体中对TiB2-C复合阴极电解膨胀性能的研究表明,与半石墨质阴极在相同电解质体系、相同电解工艺条件下所测得的阴极电解膨胀率相比,沥青、呋喃、酚醛、环氧基TiB2-C复合阴极均表现出了较小的电解膨胀率,降幅最少为9.0%,最高则达到了56.4%;张凯等[21]的研究也发现,沥青、呋喃、酚醛、环氧基TiB2-C复合阴极的年均腐蚀率分别为7.29、5.84、2.31和2.63 mm/a,与半石墨质阴极的年均腐蚀率相比(15 mm/a),均有较大幅度的降低。这使得采用TiB2-C复合阴极来解决含钾低温电解质熔体对阴极所带来的不利影响成为可能。
为了推动惰性电极系统的工业化应用,加快含钾低温电解质体系的研发,深化对碱金属在该体系中的析出及其在阴极中渗透迁移行为的认识,本文作者首先以沥青基TiB2-C复合阴极为研究对象,考察其在不同组成电解质熔体中电解时,碱金属K、Na在其中的渗透迁移行为。在此基础上,综合采用电化学测试方法研究KF-NaF熔体和K3AlF6/Na3AlF6基熔体中碱金属K、Na的析出行为及阴极膨胀机理。
1 实验
1.1 碱金属渗透迁移行为
2.1.1 实验原料
TiB2-C复合阴极配方如表1所示。TiB2粉末:平均粒度12 μm;石油焦:106~270 μm。试样的规格及其制备方法与文献[22]中所报道的一致。
表1 沥青基TiB2-C复合阴极配方
Table 1 Formula of pitch based TiB2-C composite cathode

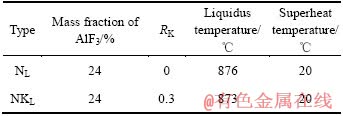
电解实验所用的化学试剂为:K3AlF6(分析纯),Na3AlF6(分析纯),Al2O3(分析纯)和AlF3(分析纯)。电解质组成,熔体初晶温度和过热度见表2,表2中的RK为电解质中钾冰晶石含量(%)与钾冰晶石和钠冰晶石总含量(%)之比;NL为Na3AlF6基电解质熔体(RK=0);NK为K3AlF6基电解质熔体(RK=0.3)。
表2 冰晶石基电解质熔体组成及相关参数
Table 2 Composition and relative parameters of cryolite based electrolysis bath
1.1.2 分析方法
待测试样被置于一垂直管式炉内的高纯石墨制电解槽中,试样与电解槽之间用刚玉片隔开,试样浸入电解质的深度为25 mm。在每种组成的电解质熔体中均进行五次电解实验,电解时间分别为5、15、30、60、120 min。实验所用氧化铝的浓度为相应电解质的饱和氧化铝浓度,电流密度(ρCD)为0.8 A/cm2,实际电解温度等于电解质的初晶温度(tL)与过热度(tS)之和。整个实验是在高纯氩气保护性气氛下进行的。采用改进型阴极电解膨胀率测试仪,记录电解过程中阴极试样的电解膨胀率。电解完成后,将试样取出,并沿其径向方向剖开,其剖面距试样底部约10 mm,采用NORAN VANTAGE4105型X射线能谱仪对试样剖面进行元素线扫描和面扫描分析。
1.2 循环伏安
1.2.1 实验试剂
实验电解质组成及熔体温度如表2和3所列。表3中的NK0、NK10、NK20、NK30分别表示由NaF和KF混和组成的电解质体系(其RK分别为0、0.1、0.2、0.3)。所使用的无水KF是由KF·2H2O脱水制得。首先在刚玉坩埚内于120 ℃缓慢加热脱去吸附水,然后将KF研磨成粉末状,加热至400 ℃恒温12 h烘干脱去结晶水。而NaF、Na3AlF6、K3AlF6、AlF3、Al2O3等在使用前也均经120 ℃下恒温12 h烘干处理。
表3 氟化物熔体组成及相关参数
Table 3 Composition of fluoride based bath

1.2.2 电化学测试装置
采用三电极系统,循环伏安法研究电极过程。实验槽采用d 70 mm×100 mm的石墨坩埚,外置d 120 mm×250 mm的刚玉套筒。工作电极采用直径5 mm的光谱纯石墨棒。使用前,磨至镜面、超声波清洗、烘干。对电极采用高纯石墨,并设计成特殊的L型,如图1所示(CE为对电极,WE为工作电极,RE为参比电极)。参比电极选用直径为1 mm的裸铂电极,其稳定性已得到验证[23]。电化学测试所使用的仪器为上海辰华仪器有限公司生产的CHI660b恒电位仪。
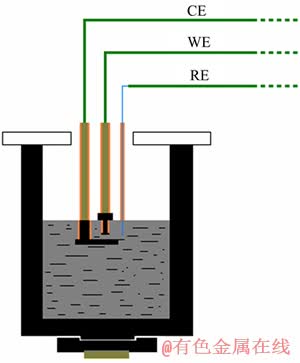
图1 电化学测试装置示意图
Fig. 1 Schematic diagram of apparatus for electrochemical measurement
2 结果与讨论
2.1 阴极中碱金属的渗透迁移行为
以传统阴极为研究对象,ADHOUM等[24]在1 025 ℃的条件下,研究熔融NaF熔体中碱金属钠对石墨阴极的电化学插层,证实钠插层进入石墨阴极的两种机理:电解过程中阴极表面所析出的碱金属Na,一部分插层进入石墨层间,另一部分则沉积在阴极材料的孔隙当中。进入石墨层间的碱金属Na随后发生重排,形成层间化合物;而材料孔隙当中存在的碱金属Na会通过扩散,到达插层位置,最终同样会形成插层化合物。LIU等[25]研究1 163 K条件下,熔融KF熔体中碱金属钾对石墨阴极的电化学插层,发现K仅仅插层进入了石墨层间。然而,单组份电解质熔体与复合电解质熔体差异较大,尤其对于含K复合低温电解质体系而言,碱金属K、Na的析出及其渗透迁移行为将会有所不同。因此,有必要对含钾复合低温电解质熔体中K、Na的析出、插层及其对阴极的渗透迁移行为进行研究。
图2和3所示分别为不同电解时间条件下沥青基TiB2-C复合阴极在Na3AlF6和K3AlF6 基电解质熔体中电解后阴极剖面元素线扫描结果。图中灰黑色斑点区域为碳质骨料颗粒,其余部分为TiB2和粘结剂的混合区域。之所以将碳质骨料的粒度控制在106~270 μm,就是为了便于考察电解后碱金属对阴极中不同组分的渗透情况。图4所示为电解后复合阴极中TiB2的形貌和EDS分析。
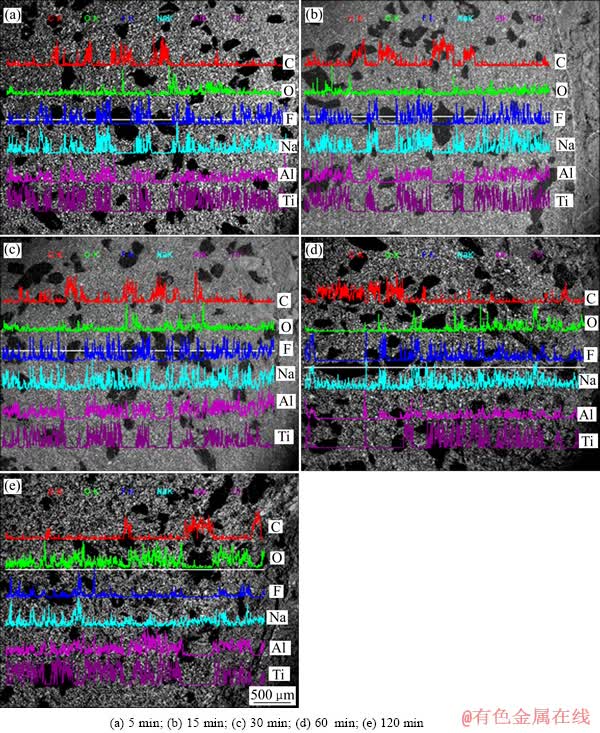
图2 Na3AlF6熔体中电解后沥青基TiB2-C复合阴极电解后剖面元素线扫描分析
Fig. 2 Elemental line scanning of cross sections for specimens after electrolysis in Na3AlF6 based bath at different electrolysis times
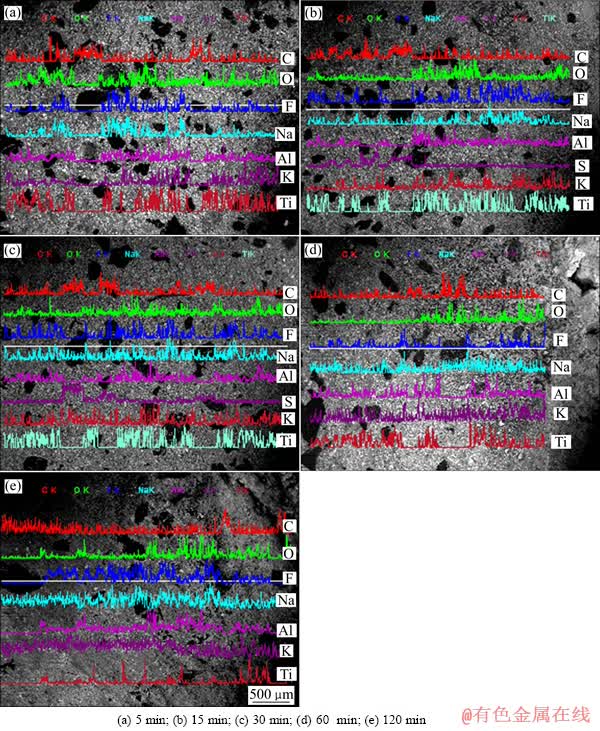
图3 K3AlF6熔体中电解后沥青基TiB2-C复合阴极电解后剖面元素线扫描分析
Fig. 3 Elemental line scanning for cross sections of specimens after electrolysis in K3AlF6 based bath at different electrolysis times
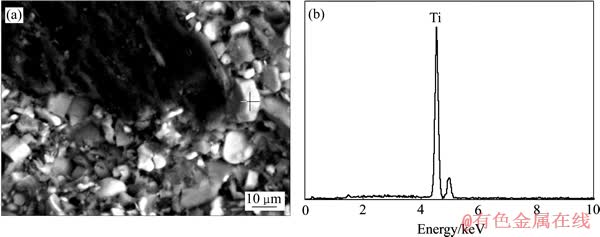
图4 电解后复合阴极中TiB2的形貌和EDS分析
Fig. 4 Morphology(a) and EDS analysis(b) of TiB2 grain in composite cathode after electrolysis
从图2中可以看出,电解5 min之后,元素F和Na均渗透进入阴极中TiB2和粘结剂的混合区域,但碳质骨料中几乎没有任何元素的渗入,从图4更加验证了这一观点。因此,电解5 min后,渗入TiB2和粘结剂混合区域的元素F和Na均存在于粘结剂结焦碳和热解过程中所形成的孔隙中。当电解进行15 min之后,除粘结剂中渗入元素F和Na之外,碳质骨料颗粒中也渗入了少量的元素Na,但其中仍然没有元素F的渗入。元素Na和F没有同时渗透进入碳质骨料中,说明碳质骨料中所渗入的元素Na是以碱金属原子形式渗透进入其中并形成了C-Na插层化合物。当电解时间延长至30 min和60 min之后,这种现象表现的更为明显。最终,电解120 min后,可以清楚地看到,无论是碳质骨料还是TiB2与粘结剂的混合区域中均渗入了元素Na,但渗入碳质骨料中的元素F仍然很少,进一步说明,元素Na是以碱金属原子的形式渗入碳质骨料中并形成了相应的C-Na插层化合物,最终引起阴极膨胀的,这一点从图5中可以得到证实。图3所示的结果与图2的类似,碱金属首先随电解质一同渗透进入阴极的孔隙当中,随后又逐渐渗透进入粘结剂结焦碳中,随着电解的进行,最终渗入阴极碳质骨料颗粒中。同时,随着碱金属的不断渗入,阴极电解膨胀率不断增大,并随阴极中碱金属浓度的饱和而达到平衡。
图5所示为不同电解时间条件下沥青基TiB2-C复合阴极在Na3AlF6 和K3AlF6 基电解质熔体中的电解膨胀率曲线。从图5中可以看出,电解2 h后,Na3AlF6 基电解质熔体中的阴极电解膨胀率为0.66%,而K3AlF6 基电解质熔体中的阴极电解膨胀率为1.35%,为Na3AlF6 基熔体中测试结果的2.05倍,说明钾冰晶石的添加会引起阴极更大的电解膨胀率。此外,还可以看出,在这两种电解质熔体中进行电解时,在电解时间仅为5 min时,阴极便发生了膨胀,随着电解时间的不断延长,膨胀量逐渐增大,结合图2和3所得的结果可以推断,阴极的电解膨胀率是由粘结剂结焦碳和碳质骨料的膨胀共同引起的。从图2和3中可以看出,在电解时间为5 min的条件下,阴极碳质骨料中几乎没有元素K、Na的渗透,而在粘结剂相中,则检测出有元素K、Na的渗入。这部分K、Na除一部分由存在于粘结剂孔隙中的电解质所引入以外,另一部分则是粘结剂结焦碳插层化合物中的K和Na,因为从电解膨胀率的测试结果可以看出,电解5 min后,阴极已发生了膨胀,这只能是由粘结剂结焦碳中的插层化合物所引起,而这些插层化合物也只能是由渗透进入阴极的碱金属K、Na与粘结剂结焦碳反应而生成的。
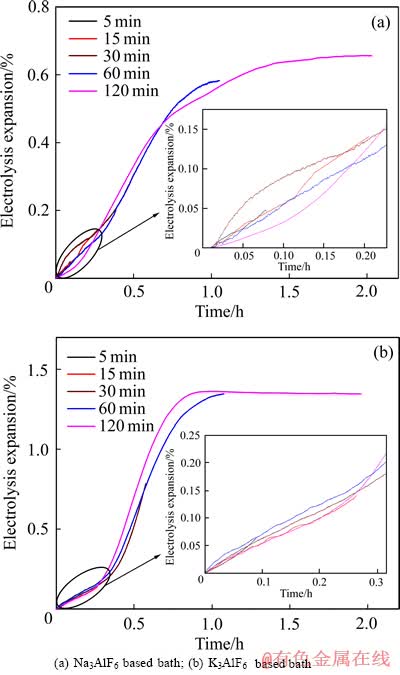
图5 不同电解质熔体中TiB2-C复合阴极的电解膨胀率
Fig. 5 Electrolysis expansion of TiB2-C composite cathodes in different cryolite baths
TiB2-C复合阴极中碱金属的渗透迁移过程可以被看作是一个扩散过程,其所渗入的碱金属的量与阴极电解膨胀率成正比,根据ZOLOCHEVSKY等[26]所提出的模型,可以计算出电解过程中碱金属在阴极中的扩散系数。表4所列为不同电解质熔体中,TiB2-C复合阴极中碱金属的扩散系数。在Na3AlF6基电解质熔体(NL)中,碱金属的扩散系数为2.82×10-5 cm2/s;而在K3AlF6基电解质熔体(NK)中,碱金属的扩散系数为2.86×10-5 cm2/s,与Na3AlF6基电解质熔体中碱金属的扩散系数相差不大。K、Na同属碱金属元素,性质较为相似,其在阴极中的扩散系数主要受电解温度的影响。在Na3AlF6和K3AlF6基熔体中,电解温度分别为896 ℃和893 ℃,相差不大,这就致使碱金属在阴极中的扩散系数相差不大。
表4 不同电解质熔体中碱金属的扩散系数及相关参数
Table 4 Parameters and diffusion coefficient for samples with various baths

图6所示为不同电解时间条件下沥青基TiB2-C复合阴极在K3AlF6 基熔体中电解后阴极剖面元素面扫描结果。与图3类似,BSE图中的灰黑色区域为碳质骨料颗粒,其余部分为TiB2和粘结剂的混合区域。从图6中可以看到,当电解进行15 min后,阴极粘结剂相中便有F、K、Na的渗入,但碳质骨料中,除了少量K 、Na的渗入外,几乎没有F元素的渗入。随着电解时间的不断延长,即使在电解120 min后,阴极碳质骨料中仍仅有元素K、Na的渗入而并无明显的元素F的渗入。此外,还可以看出,K的渗透迁移路径虽然与Na的类似,但却表现出了较强的渗透力。如图6(c1)、(c2)、(c3)、(c4)所示,当电解时间为120 min时,元素K对阴极各组分的渗透几乎没有区别,并且渗入量明显大于Na。与元素F的面扫面结果进行对比后可知,渗入阴极碳质骨料当中的这部分K,应是以C-K插层化合物的形式存在,因为如果这部分K是由电解质带入的,在面分析结果中,其应该与F在同一区域出现。

图6 不同电解时间条件下TiB2-C复合阴极在K3AlF6熔体中电解后的形貌及剖面元素面扫描
Fig. 6 Morphologies and elemental map scanning for cross sections of specimens after electrolysis under different time in K3AlF6 based bath
2.2 熔体中碱金属(K、Na)的电极过程
碱金属K、Na的析出及其渗透迁移行为与其在熔体中的电极过程密切相关,了解其电极过程能为深化认识碱金属插层化合物的形成机制以及具体渗透迁移行为提供有力支撑。
2.2.1 氟化物熔体中碱金属(K、Na)的电极过程
在冰晶石熔体中,离子结构较为复杂,直接在其中进行电化学测试,所得结果不容易解析。因此,首先在较为简单的熔融氟化物熔体中进行电化学测试。在熔融氟化物熔体中,碱金属会发生两方面的作用,一方面,其会在熔体中发生溶解反应,生成相应的偏晶体。另一方面,由于所使用熔体的温度均高于碱金属K、Na的沸点,因此,在测试过程中,碱金属会发生挥发。这两种现象均会对循环伏安的测试结果产生影响,导致测试结果与实际情况发生偏差。为了避免这种影响,循环伏安测试必须选择一个合适的扫描速率。通过文献调研[25, 27-28],本文作者在进行循环伏安测试时,选择的扫描速率为100 mV/s。在此基础上,采用钼丝作为工作电极进行验证试验,结果如图7所示。

图7 NaF熔体中钼电极上的循环伏安曲线
Fig. 7 Cyclic voltammograms recorded on molybdenum electrode in molten NaF (Potential scan rate: 100 mV/s)
从图7中可以看出,由于使用了纯NaF,熔体中Na+的活度较高,因而在正向扫描的阴极过程中,Na+的还原反应得到强化,反应强度增大,从而表现出较大的还原电流。而在进行反向扫描时,阳极过程却没有表现出明显的氧化峰。这主要是因为在进行循环伏安测试过程中,所选择的扫描速率不高,这使得在阴极过程中还原生成的Na有足够的时间发生溶解或挥发,这便避免了碱金属Na的氧化对实验结果的影响,说明在进行循环伏安测试时,选择扫描速率为100 mV/s是合适的。
图8所示为KF的添加量分别为10%和30%(摩尔分数)时氟化物熔体中石墨电极的循环伏安曲线。由图8可以看出,随着KF添加量的增大,第二个峰的电流也逐渐增大,在排除了阳极过程碱金属的氧化之后,可以推断其对应于C-K插层化合物的氧化反应。故第一个峰对应为C-Na插层化合物的氧化反应。与以钼丝为工作电极时所得结果相比较,可以看出无论使用何种组成的电解质熔体,阴极过程的还原电流都很大,这一方面是由于熔体中具有较高的碱金属离子活度;另一方面则是因为,实验所使用的工作电极为光谱纯石墨,在正向扫描的阴极过程中,碱金属一旦析出,便会立即被吸收,生成相应的插层化合物。这一点,从反向扫描的阳极过程来看更为明显。如图8所示,阳极过程出现了两个明显的氧化峰,与图7相比,可以看出,该氧化峰对应于石墨层间化合物的氧化反应。因为在扫描速率为100 mV/s的测试条件下,碱金属的氧化已被排除。石墨属于六方晶系,具有特殊的层状结构。层面内碳原子之间是强的σ键,具有极强的键合能,而层间的碳原子之间仅以微弱的范德华力相结合[29],因此,碱金属原子能轻易地突破层间结合力而插入石墨层间,形成石墨层间化合物。
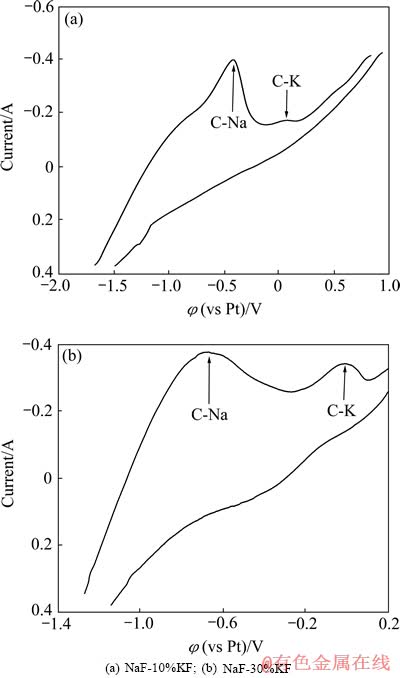
图8 氟化物熔体中石墨电极循环伏安曲线
Fig. 8 Cyclic voltammograms recorded on graphite in molten fluoride (Potential scan rate: 100 mV/s)
与所示阳极过程中C-Na插层化合物所对应的氧化峰相比,C-K插层化合物所对应的氧化峰的位置向正的方向偏移了。此外还可以看到,就阴极过程而言,图8(a)和(b)所示的结果较为类似。综合上述两方面的现象可以看出,阴极过程中碱金属K与Na发生了共析出,并表现出了类似的插层所用。而阳极过程所表现出的现象则说明,与C-Na插层化合物相比,在反向扫描的阳极过程中,C-K插层化合物更难以被氧化,K嵌入进石墨层间所形成的C-K插层化合物具有更高的稳定性,因此会对阴极产生更大的影响,这印证宏观上阴极电解膨胀率和耐腐蚀性能的测试结果,即与纯钠体系相比,含钾电解质体系对阴极的破坏力更强。
2.2.2 冰晶石(K3AlF6/Na3AlF6)-Al2O3熔体中碱金属的电极过程
在上述测试结果的基础上,研究接近于工业实际的冰晶石(K3AlF6/Na3AlF6)-Al2O3熔体中电极过程更有意义。图9所示为以光谱纯石墨为工作电极所进行的循环伏安测试结果。
从图9(a)中可以看出,在正向扫描的阴极过程中从φ=-1.4 V开始出现一个明显的阴极峰。测试中所使用的电解质熔体为Na3AlF6-Al2O3,Na的析出电势比Al低250 mV,因而,图9(a)中正向扫描的阴极过程中所出现的阴极峰主要为析出铝的反应,正好与反向扫描时所出现的阳极峰对应。同时又可以看出,Na与Al的析出电势相差不大,在阴极极化条件下,Na与Al便可能发生共沉积。然而这一点在图9(a)中却没有表现出来,这主要是因为,在Na3AlF6-Al2O3熔体中,阴极和阳极过程主要为铝的析出与氧化,其所对应的阴极电流和阳极电流都比较大,Na及其插层化合物所对应的氧化还原峰便会被铝的氧化还原峰所掩盖,导致图9(a)中并没有出现C-Na插层化合物的氧化峰及Na的还原峰。从图9(b)中可以看出,阴极过程从φ=-1.5 V开始出现一个明显的还原峰。考虑到所使用电解质的组成,可以推断阴极过程主要为Al、K和Na的共析出。在反向扫描的阳极过程中,出现了两个氧化峰,与图9(a)相比,结合氟化物体系中循环伏安的测试结果,可以推断,在φ=-0.7 V处所出现的阳极峰与C-K插层化合物的氧化对应,进一步证实,C-Na插层化合物所对应的氧化峰之所以没有出现,主要是因其被Al的氧化峰掩盖所致。在冰晶石-氧化铝熔体中,Na的析出电势比铝低250 mV,而K的析出电势又比Na低140 mV。图9(b)所示氧化过程中C-K插层化合物所对应的氧化峰的出现说明在阴极过程中,一定存在K的析出,而Na的析出电势处在Al和K之间,因此,循环伏安测试过程中,必然存在Na的析出及其所对应C-Na插层化合物的氧化。此外,还可以看出,与Al及C-Na插层化合物所对应的氧化峰相比,C-K插层化合物所对应氧化峰的位置较正,说明虽然Al、K、Na在阴极过程中发生共沉积,但C-K插层化合物的稳定性更高,插入石墨层间形成插层化合物的K很难脱出,K的原子半径比Na的大37.2 pm。这些因素共同作用导致相同条件下渗透进入阴极的K与C反应所生成的C-K插层化合物会对阴极造成更大的影响,引起阴极更大的电解膨胀率,与前述分析结果对应。

图9 冰晶石熔体中石墨电极上的循环伏安曲线
Fig. 9 Cyclic voltammograms recorded on graphite in molten cryolite based bath (Potential scan rate: 100 mV/s)
3 结论
1) 电解条件下,在TiB2-C复合阴极中,碱金属K、Na首先随电解质一同渗透进入阴极的孔隙当中,随后又渗透进入粘结剂结焦碳中,随着电解的进行,最终逐渐渗透进入阴极碳质骨料颗粒中,碱金属K、Na不会渗透进入TiB2颗粒中,K比Na有着更强的渗透力。渗透进入粘结剂和碳质骨料中的碱金属K、Na均会引起阴极的电解膨胀,且随阴极中碱金属浓度的饱和而达到平衡。
2) 冰晶石基熔体中,在扫描速率为100 mV/s的条件下,以Pt丝为参比电极,高纯石墨上所进行的循环伏安测试表明,正向扫描的阴极过程中,在电位为-1.5~-1.4 V的范围内,出现明显的Al、K和Na的共析出峰。虽然在反向扫描过程中,C-Na插层化合物的氧化峰被Al的氧化峰所掩盖,但在-0.7 V处所出现的C-K插层化合物的氧化峰,一方面证实了阴极过程中一定存在K、Na的析出及其所对应插层化合物的形成,另一方面还印证了C-K插层化合物具有更高的稳定性,插入石墨层间形成插层化合物的K很难脱出,其对阴极有着较强的破坏力。
REFERENCES
[1] VERBRUGGE M W, KRAJEWSKI P, SACHDEV A K. Challenges and opportunities relative to increased usage of aluminum within the automotive industry[C]// JOHNSON J A. Light metals 2010. Seattle, USA: TMS, 2010: 3-11.
[2] JAMES W E, HALVOR K. Sustainability, climate change, and greenhouse gas emissions reduction: Responsibility, key challenges, and opportunities for the aluminum industry[J]. JOM, 2008, 60(8): 25-31.
[3] 刘业翔. 有色金属冶金基础研究的现状及对今后的建议[J]. 中国有色金属学报, 2004, 14(l): 21-24.
LIU Ye-xiang. Current status and future proposals of fundamental research in nonferrous metallurgy[J]. Transactions of nonferrous metals society of china, 2004, 14(1): 21-24.
[4] 邱竹贤. 21世纪伊始铝电解工业的新进展[J]. 中国工程科学, 2003, 5(4): 41-46.
QIU Zhu-xian. New progress of aluminum industry in the beginning of 21st century[J]. Engineering Science, 2003, 5(4): 41-46.
[5] 刘业翔, 李 劼. 现代铝电解[M]. 北京: 冶金工业出版社, 2008: 586-589.
LIU Ye-xiang, LI Jie. Modern aluminum electrolysis[M]. Beijing: Metallurgical Industry Press, 2008: 586-589.
[6] ALCOM T R, STEWART D V, TABEREAUX A T. Pilot reduction cell operation using TiB2-G cathodes[C]. BLCKERT C M. Light Metals 1990. Warrendale, USA: TMS, 1990: 413-418.
[7]  Xiao-jun, XU Jian, LI Jie, LAI Yan-qing, LIU Ye-xiang. Thermal-treated pitches as binders for TiB2/C composite cathodes[J]. Metallurgical and Materials Transactions A, 2012, 43(1): 219-227.
Xiao-jun, XU Jian, LI Jie, LAI Yan-qing, LIU Ye-xiang. Thermal-treated pitches as binders for TiB2/C composite cathodes[J]. Metallurgical and Materials Transactions A, 2012, 43(1): 219-227.
[8] HOU Jin-long,  Xiao-jun, ZHANG Hong-liang, LAI Yan-qing, LI Jie. Furan resin and pitch blends as binders for TiB2-C cathodes[C]// LINDSAY S J. Light Metals 2011. San Diego, USA: TMS, 2011: 1117-1121.
Xiao-jun, ZHANG Hong-liang, LAI Yan-qing, LI Jie. Furan resin and pitch blends as binders for TiB2-C cathodes[C]// LINDSAY S J. Light Metals 2011. San Diego, USA: TMS, 2011: 1117-1121.
[9] HEIDARI H, ALAMDARI H,  D, SCHULZ R. Pressureless sintering of TiB2-based composites using Ti and Fe additives for development of wettable cathodes[C]// LINDSAY S J. Light Metals 2011. San Diego, USA: TMS, 2011: 1111-1116.
D, SCHULZ R. Pressureless sintering of TiB2-based composites using Ti and Fe additives for development of wettable cathodes[C]// LINDSAY S J. Light Metals 2011. San Diego, USA: TMS, 2011: 1111-1116.
[10] CRAIG B. Next generation venial electrode cells[J]. JOM, 2001, 53(5): 39-42.
[11] JACOBS T B, BROOKS R. Electrolytic reduction of aluminum: United States, 5279715[P]. 1994.
[12] 高炳亮. 低温铝电解新研究[D]. 沈阳: 东北大学, 2003: 3-10.
GAO Bing-liang. New studies on low-temperature aluminum electrolysis[D]. Shenyang: Northeastern University, 2003: 3-10.
[13] 王家伟. Na3AlF6-K3AlF6-AlF3体系的初晶温度、Al2O3溶解能力及NiFe2O4基惰性阳极低温电解腐蚀研究[D]. 长沙: 中南大学, 2008: 7-14.
WANG Jia-wei. Research on liquidus temperature and Al2O3 dissolving ability of Na3AlF6-K3AlF6-AlF3 system and corrosion resistance of NiFe2O4 based inert anodes in low temperature aluminum electrolysis[D]. Changsha: Central South University, 2008: 7-14.
[14] GALASIU I, GALASIU R, THONSTAD J. Inert anodes for aluminium electrolysis[M]. Düsseldorf: Aluminium-Verlag, 2007: 23-24.
[15] NAAS TYKE, OYE H A. Interactions of alkalimetal with cathode carbon[C]. ECKERT C E. Light Metals 1999. San Diego, USA: TMS, 1999: 193-198.
[16] LI Jie, FANG Zhao, LAI Yan-qing,  Xiao-jun, TIAN Zhong-liang. Electrolysis expansion performance of semigraphitic cathode in [K3AlF6/Na3AlF6]-AlF3-Al2O3 bath system[J]. Journal of Central South University of Technology, 2009, 16(3): 422-428.
Xiao-jun, TIAN Zhong-liang. Electrolysis expansion performance of semigraphitic cathode in [K3AlF6/Na3AlF6]-AlF3-Al2O3 bath system[J]. Journal of Central South University of Technology, 2009, 16(3): 422-428.
[17] MORTEN S, OYE H A. Cathodes in aluminium electrolysis[M]. Düsseldorf: Aluminium-Verlag, 1994: 66-73.
[18] MCMINN C J. Review of RHM cathode development[C]// CUTSHALL E R. Light Metals 1992. San Diego, USA: TMS, 1992: 419-425.
[19] PAWLEK R P. Cathodes wettable by molten aluminum for aluminumelectrolysis cells[J]. Aluminium, 1990, 66(8): 573-582.
[20] FANG Zhao, LI Jie,  Xiao-jun, LAI Yan-qing TIAN Zhong-liang. Electrolysis expansion Performance of TiB2-C composite cathode in [K3AlF6/Na3AlF6]-AlF3-Al2O3 bath melts[C]// JOHNSON J A. Light Metals 2010. Seattle, USA: TMS, 2010: 901-906.
Xiao-jun, LAI Yan-qing TIAN Zhong-liang. Electrolysis expansion Performance of TiB2-C composite cathode in [K3AlF6/Na3AlF6]-AlF3-Al2O3 bath melts[C]// JOHNSON J A. Light Metals 2010. Seattle, USA: TMS, 2010: 901-906.
[21] 张 凯, 方 钊, 吕晓军, 赖延清, 李 劼. 低温电解质[K3AlF6/Na3AlF6]-AlF3-Al2O3熔体中TiB2-C复合阴极的耐腐蚀性能[J]. 中南大学学报, 2011, 42(3): 588-594.
ZHANG Kai, FANG Zhao,  Xiao-jun, LAI Yan-qing, LI Jie. Corrosion resistance of TiB2-C cathode composites for aluminum electrolysis in [K3AlF6/Na3AlF6]-AlF3-Al2O3 melts[J]. Journal of Central South University of Technology, 2011, 42(3): 588-594.
Xiao-jun, LAI Yan-qing, LI Jie. Corrosion resistance of TiB2-C cathode composites for aluminum electrolysis in [K3AlF6/Na3AlF6]-AlF3-Al2O3 melts[J]. Journal of Central South University of Technology, 2011, 42(3): 588-594.
[22] LI Jie, FANG Jing, LI Qing-yu, LAI Yan-qing. Effect of TiB2 content on resistance to sodium penetration of TiB2/C cathode composites for aluminum electrolysis[J]. Journal of Central South University of Technology, 2004, 15(1): 400-404.
[23] 谢 中, 刘业翔. 高温氯化物熔体参比电极[J]. 中国有色金属学报, 1998, 8(4): 109-113.
XIE Zhong, LIU Ye-xiang. Reference electrode in molten chlorides at high temperature[J]. Transactions of Nonferrous Metals Society of China, 1998, 8(4): 109-113.
[24] ADHOUM N, BOUTEILLON J, DUMAS D, POIGNET J C. Electrochemical insertion of sodium into graphite in molten sodium fluoride at 1 025 ℃[J]. Electochimica Acta, 2006, 51(25): 5402-5406.
[25] LIU Dong-ren, YANG Zhan-hong, LI Wang-xing, QIU Shi-lin, LUO Ying-tao. Electrochemical intercalation of potassium into graphite in KF melt[J]. Electrochimica Acta, 2010, 55(3): 1013-1018.
[26] ZOLOCHEVSKY A, HOP J G, SERVANT G, FOOSNAES T, OYE H A. Rapoport-Samoilenko test for cathode carbon materials I. Experimental results and constitutive modelling[J]. Carbon, 2003, 41(3): 497-505.
[27] CASTRILLEJO Y, BERMEJO M R, AROCAS P D, MARTINEZ A M, BARRADO E. The electrochemical behavior of the Pr(III)/Pr redox system at Bi and Cd liquid electrodes in molten eutectic LiCl-KCl[J]. Journal of Electroanalytical Chemistry, 2005, 579(2): 343-358.
[28] SANCHEZ S R, PICARD G S. Solubility and diffusion of metallic iron in liquid Bi metal at 450 ℃[J]. Electrochemistry Communications, 2004, 6(9): 944-954.
[29] 传秀云. 石墨层间化合物GICs的形成机理探讨[J]. 新型碳材料, 2000, 15(1): 52-56.
CHUAN Xiu-yun. Formation mechanisms of graphite intercalation compounds[J]. New Carbon Materials, 2000, 15(1): 52-56.
(编辑 李艳红)
基金项目:西安建筑科技大学青年科技基金资助项目(QN1221);西安建筑科技大学人才科技基金资助项目(RC1132)
收稿日期:2013-02-26;修订日期:2013-04-28
通信作者:方 钊,讲师,博士;电话:029-82202923;E-mail: fangzhao889@126.com