
Effect of cooling rate and composition on microstructures and
properties of Zn-Mg alloys
WANG Xiang(王香), LU Hong-mei(陆红梅), LI Xin-lin(李新林),
LI Li(李莉), ZHENG Yu-feng(郑玉峰)
Center for Biomedical Materials and Engineering, Harbin Engineering University, Harbin 150001, China
Received 15 July 2007; accepted 10 September 2007
Abstract: The Zn-Mg alloys with Mg additions of 35%, 40% and 45%(mass fraction) were prepared by conventional casting method, with the aim to develop new biodegradable materials. The effects of cooling rate and composition on the microstructures, hardness and corrosion resistance were studied by XRD, SEM, microhardness and corrosion testing techniques. The corrosion behaviors of experimental alloys in simulated body fluids were analyzed. The results show that the amount of the petal-like MgZn2 phase decreases, as well as the hardness of the alloys, but that of the polygonal MgZn2 phase increases with the increase of Mg content when the cooling rate is constant. When the alloy composition is constant, the MgZn2 phase changes easily from petal-like to polygon, and the hardness decreases with the decrease of the cooling rate.
Key words: Zn-Mg alloys; microstructure; corrosion behavior
1 Introduction
In the area of biomaterials, biodegradable implant materials have attracted more attention because they can be gradually dissolved, absorbed, consumed or excreted in the human body[1-2]. Biodegradable materials made of polymers can only be used in limited area because of their poor mechanical properties[3]. But metallic biomaterials are still playing an important role in repairing or replacement of bone tissue[4]. Metals are more suitable for load-bearing applications compared with ceramics or polymeric materials due to their combination of high mechanical strength and fracture toughness. However, most of metallic biomaterials have poor biodegradability and release toxic metallic ions and/or particles in the process of corrosion or wear[5-6]. So it is important to find an alloy that is non-toxic to human bodies and has adjustable corrosion rates. Zinc has a high corrosion resistance and is an essential micronutrient that plays fundamental housekeeping roles in physiology, cellular metabolism and gene expression[7]. It’s expected to be developed as biodegradable implant material with appropriate corrosion rate in simulated body fluids via adding appropriate alloy elements. Magnesium is essential to human metabolism, it can be easily degraded and absorbed[8]. Although a few papers about the intermetallic phases of the Zn-Mg alloy have been published[9-13], there is little research on the effects of cooling rate and alloying composition on the microstructure and properties. Especially, the biomedical application of Zn-Mg alloy has rarely been reported. The main objective of the present work is to try to develop a new type of biodegradable material by studying the microstructure and properties of Zn-Mg alloys.
2 Experimental
The Zn-xMg(x=35, 40, 45) alloys were prepared by melting constituent elements (Zn, 99.995%; Mg, 99.50%) in an induction furnace under a gas atmosphere of CO2+0.5% SF6. To ensure its chemical homogeneity, the melt was mechanically stirred for 6 min, held at approximately 700 ℃ for 20 min, and then was cast into cylinder steel moulds with different diameters of 50, 25 and 10 mm.
The alloys samples were etched with a solution of 4%(volume fraction) hydrochloric acid and ethyl alcohol. Phase identification was carried out via X-ray diffraction (XRD) using Rikagu D/max-RB diffractometer. The FEI Sirion scanning electron microscope(SEM) equipped with energy-dispersive X-ray spectrometer (EDS) was used to analyze the microstructure. The microhardness of the alloys was examined with hardness instrument (HV-1000). Corrosion resistance tests, including immersion corrosion and potentiodynamic polarization measurement, were carried out in Hanks solutions[14] as simulated body fluid(SBF). The treated samples were weighed and then immersed in SBF solutions at room temperature for a certain time. The pH values of the SBF solutions were examined intermittently. The electrochemical property of the alloys was tested in SI1287 electrochemical testing system at 37 ℃ with saturated calomel electrode(SCE) as the reference electrode and platinum as auxiliary electrode. The scanning rate was 1 mV/s.
3 Results and discussion 3.1 Phase composition
Fig.1 shows the X-ray diffraction patterns of Zn-Mg alloys. It can be seen that there are MgZn2, MgZn, Mg2Zn3 and Mg7Zn3 phases in the three alloys.

Fig.1 X-ray diffraction patterns of Zn-Mg alloys
3.2 Microstructure of Zn-Mg alloy
3.2.1 Effect of composition on microstructure
Fig.2 shows the microstructures of Zn-Mg alloys at constant cooling rate; all samples were cut from ingot with diameter of 50 mm. It can be seen from Fig.2(a) that there are some hexagonal petal-like crystals, and the bigger petal-like structure represents a symmetrical hexagonal structure with a clear geometrical center. Fig.2(b) shows that the amount and dimension of the petal-like crystals decrease and some polygonal crystals emerge. It can be seen from Fig.2(c) that the petal-like structure disappears and the precipitate phases are connected with each other.
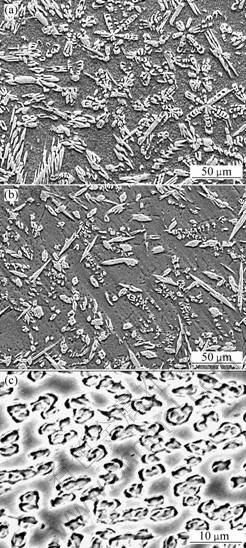
Fig.2 Microstructures of Zn-Mg alloys: (a) Zn-35Mg; (b) Zn-40Mg; (c) Zn-45Mg
Based on the Zn–Mg phase diagram[9], there are five intermetallic phases in the Zn–Mg system, namely Mg7Zn3, MgZn, Mg4Zn7, MgZn2 and Mg2Zn11. The Mg7Zn3 phase, existing at temperatures above 325 ℃, has an orthorhombic structure[10]. The crystal structure of the MgZn2 phase is hexagonal[11]. The Mg2Zn11 phase has a cubic structure[12]. The Mg4Zn7 phase has a base-centred monoclinic structure, rather than the triclinic structure assumed by Gallot and Graf[13].
According to the above mention, assisting with EDS identification and XRD patterns, the petal-like and hexagonal crystals should be MgZn2, the rod and needle-like crystals are Mg2Zn3 in Figs.1(a) and (b). The flaking gray precipitated phase in Fig.1(c) is Mg2Zn3.
3.2.2 Effect of cooling rate on microstructure
Fig.3 shows the microstructures of Zn-35Mg alloy at different cooling rates. It can be seen that the slower the cooling rate is, the coarser the MgZn2 phase is, and the more easily the morphology evolutes from petal-like to polygon. The petal-like morphology of the primary and medium-term phases is remained after rapid cooling. At the slow cooling rate, the crystals grow sufficiently. Exposure in the high-temperature melt for a long time, the petal-like structure is broken and grows up into polygonal structure under the action of interface-energy.

Fig.3 Microstructures of Zn-35Mg samples cut from ingots with different diameters: (a) 10 mm; (b) 25 mm; (c) 50 mm
3.3 Microhardness
Fig.4 shows the influence of the composition and cooling rate on microhardness of Zn-Mg alloys. It can be seen that the microhardness of alloys increases with the increase of cooling rate or the decrease of Mg content. The hardness increases because of the refinement of the primary phases after rapid cooling. The MgZn2 phase is one of the most common strengthening phases in Zn–Mg alloys. The quantity of MgZn2 phase decreases with the increase of Mg content, so the hardness of alloy is falling.

Fig.4 Effect of composition and cooling rate on microhardness of alloys samples cut from ingots with different diameters
3.4 Corrosion resistance properties
Fig.5 shows the pH values of the SBF solutions vary with immersion time. Samples were cut from ingot with the diameter of 25 mm. The curves indicate that pH values of the solution firstly increase rapidly then decrease slowly, and then increase again.
At the initial stage of immersion, the release of Zn2+ and Mg2+ from the samples alkalizes the solution and the passivating film forms gradually. After immersion for 90 h, the passivating film becomes compact, inhibiting further corrosion, what reduces the pH value of SBF solution. After immersion for 160 h, the passivating film is broken, and the Zn2+ and Mg2+ release again, so the pH value increases.

Fig.5 Varying curves of pH value with soaking time of Zn-Mg alloys
Polarization curves of Zn-Mg alloys at a constant cooling rate in SBF solution are shown in Fig.6; all samples were cut from ingot with diameter of 25 mm. The open circuit potentials (Ecorr) of Zn-35Mg, Zn-40Mg and Zn-45Mg alloys are -1.250 V, -1.215 V and -1.405 V, respectively. Moreover, these alloys are almost equal in the corrosion current densities (Jcorr). Zn-40Mg alloy has the best corrosion resistance among the three alloys.

Fig.6 Polarization curves of Zn-Mg alloys
4 Conclusions
1) The cooling rate and the alloy composition have a great influence on the microstructures and properties of Zn-Mg alloys. At a constant cooling rate, with the increasing of Mg content, the amount of petal-like MgZn2 phase decreases gradually, and the polygonal MgZn2 phase increases. For the same alloy, the slower the cooling rate is, the coarser the MgZn2 phase is, and the more easily the morphology evolutes from petal-like to polygon. At the same time, the hardness of the alloys decreases with the increase of cooling rate or the decrease of Mg content.
2) During the immersion of Zn-Mg alloys in the SBF, firstly the pH values of solution increase rapidly then decrease slowly, and then increase again.
3) The result of potentiodynamic polarization measurement reveals that the corrosion resistance of Zn-40Mg is the best among the three alloys.
References
[1] BRONNER F, FARACH-CARSON M C, MIKOS A G. Engineering of functional skeletal tissues[M]. London: Springer, 2007: 55–68.
[2] STAIGER M P, PIETAK A M, HUADMAI J, DIAS G. Magnesium and its alloys as orthopedic biomaterials: A review[J]. Biomaterials, 2006, 27(9): 1728–1734.
[3] SONG Guang-ling. Control of biodegradation of biocompatible magnesium alloys[J]. Corrosion Science, 2007, 49(4): 1696–1701.
[4] MIINOMI M. Recent metallic materials for biomedical applications[J]. Metallurgical and Materials Transaction A, 2002, 33(3): 477-486.
[5] DAVID A P, WINSTON W H. Acute toxicity of metal ions in cultures of osteogenic cells derived from bone marrow stromal cells[J]. J Appl Biomater, 1995, 6(2): 109-116.
[6] JACOBS J J, HALLAB N J, SKIPOR A K, URBAN R M. Metal degradation products: a cause for concern in metal-metal bearings?[J]. Clinical Orthopaedics &Related Research, 2003, 417: 139-147.
[7] DEVIRGILIIS C, ZALEWSKI P, PEROZZI G, MURGIA C. Zinc fluxes and zinc transporter genes in chronic diseases[J]. Mutation Research/Fundamental and Molecular mechanisms of Mutagenesis, 2007, 622(1/2): 84-93.
[8] SHAN Da-yong, ZHOU Wan-qiu, HAN En-hou, KE Wei. Corrosion and electrochemical behavior of AZ31D magnesium alloys in sodium chloride[J]. Trans Nonferrous Met Soc China, 2006, 16(z3): 1789-1792.
[9] MORISHITA M, YAMAMOTO H, SHIKADA S, KUSUMOTO M, MATSUMOTO Y. Thermodynamics of the formation of magnesium-zinc intermetallic compounds in the temperature range from absolute zero to high temperature[J]. Acta Materialia, 2006, 54(11): 3151-3159.
[10] HIGASHI I, SHIOTANI N, UDA M, MIZOGUCHI T, KATOH H. The crystal structure of Mg51Zn20[J]. Journal of Solid State Chemistry, 1981, 36(2): 225-233.
[11] YANG J, WANG J L, WU Y M, WANG L M, ZHANG H J. Extended application of edge-to-edge matching model to HCP/HCP (α-Mg/MgZn2) system in magnesium alloys[J]. Materials Science and Engineering A, 2007, 460/461: 296-300.
[12] LI Y B, BANDO Y, GOLBERG D. Mg2Zn11-MgO belt-like nanocables[J]. Chemical Physics Letters, 2003, 375(1/2): 102-105.
[13] GAO X, NIE J F. Structure and thermal stability of primary inter- metallic particles in an Mg–Zn casting alloy[J]. Scripta Materialia, 2007, 57(7): 655-658.
[14] ZENG Rong-chang, ZHANG Jin, HUANG Wei-jiu, DIETZEL W, KAINER K U, BLAWERT C, KE Wei. Review of studies on corrosion of magnesium alloys[J]. Trans Nonferrous Met Soc China, 2006, 16(z1): 763-771.
Corresponding author: WANG Xiang; Tel: +86-451-82518173; E-mail: wangxiang@hrbeu.edu.cn
(Edited by YANG You-ping)