钛沉积工艺制备TiN/cBN和TiC/金钢石涂层颗粒
来源期刊:中国有色金属学报(英文版)2014年第11期
论文作者:Walid M. DAOUSH Hee S. PARK Soon H. HONG
文章页码:3562 - 3570
关键词:熔盐反应;钛;沉积;立方氮化硼;金刚石;涂层;TiN;TiC
Key words:molten salt reaction; titanium; deposition; cubic boron nitride; diamond; coating; TiN; TiC
摘 要:用钛熔盐沉积及热处理工艺分别制备碳化钛涂覆的立方碳化硼颗粒(TiN/cBN)及碳化钛涂覆的金刚石颗粒(TiC/金刚石)。将cBN或金刚石颗粒分别与钛粉和KCl、NaCl和K2TiF6熔盐混合。将所得混合物在Ar气氛中加热至900 °C,然后在H2气氛中于1000 °C进行热处理。采用扫描电镜、X射线衍射和聚焦离子束技术对所制得颗粒进行表征。结果表明:cBN和金刚石颗粒表面已覆盖了纳米钛层。对Ti/cBN和TiC/金刚石涂层颗粒进行热处理后,颗粒表面沉积的Ti层与cBN和金刚石颗粒发生了原位化学反应,分别转化为钛化合物TiN和TiC。
Abstract: Cubic boron nitride particles coated by titanium nitride (TiN/cBN) as well as diamond particles coated by titanium carbide (TiC/diamond) were prepared by Ti molten salt deposition followed by heat-treatment process. cBN or diamond particles were mixed separately with Ti powders and molten salts (KCl, NaCl and K2TiF6). The mixture was heated at 900 °C under argon atmosphere. The produced particles were heat-treated under hydrogen at 1000 °C. The morphologies and chemical compositions of the produced particles were investigated by scanning electron microscopy (SEM), X-ray diffraction (XRD) and focused ion beam (FIB). The results show that the cBN and the diamond particles are coated by nano-sized Ti layers. By heat-treatment of the Ti/cBN and TiC/diamond coated particles under hydrogen atmosphere, the deposited Ti layers were interacted by the in-situ transformation reaction with the surfaces of cBN and diamond particles and converted to titanium compounds (TiN and TiC), respectively.
Trans. Nonferrous Met. Soc. China 24(2014) 3562-3570
Walid M. DAOUSH1, Hee S. PARK2, Soon H. HONG3
1. Department of Production Technology, Faculty of Industrial Education, Helwan University, Cairo 11511-11668, Egypt;
2. Institute of Industrial Technology, ILJIN Diamond Co., Ltd., Chungcheongbuk 369-824, Korea;
3. Department of Materials Science and Engineering, Korea Advanced Institute of Science and Technology, Daejon 305-701, Korea
Received 19 November 2013; accepted 2 September 2014
Abstract: Cubic boron nitride particles coated by titanium nitride (TiN/cBN) as well as diamond particles coated by titanium carbide (TiC/diamond) were prepared by Ti molten salt deposition followed by heat-treatment process. cBN or diamond particles were mixed separately with Ti powders and molten salts (KCl, NaCl and K2TiF6). The mixture was heated at 900 °C under argon atmosphere. The produced particles were heat-treated under hydrogen at 1000 °C. The morphologies and chemical compositions of the produced particles were investigated by scanning electron microscopy (SEM), X-ray diffraction (XRD) and focused ion beam (FIB). The results show that the cBN and the diamond particles are coated by nano-sized Ti layers. By heat-treatment of the Ti/cBN and TiC/diamond coated particles under hydrogen atmosphere, the deposited Ti layers were interacted by the in-situ transformation reaction with the surfaces of cBN and diamond particles and converted to titanium compounds (TiN and TiC), respectively.
Key words: molten salt reaction; titanium; deposition; cubic boron nitride; diamond; coating; TiN; TiC
1 Introduction
Diamond and cubic boron nitride (CBN) are the hardest materials, and are thermodynamically stable materials under high pressure and temperature conditions. However, it is difficult to sinter diamond and cBN by conventional sintering techniques. Polycrystalline diamond and cBN have been produced using sintering additives. The sintering conditions depend on the additives and/or binding materials, and require pressure of 6 GPa and temperature of 1500 °C. These sintering conditions are attained using ultrahigh pressure devices. In addition, when additives such as TiN, AlN and TiC, are added in the form of powders to diamond and cBN particles for solid phase sintering, their capacity to effectively act as sintering additives or as binding materials is limited [1]. To be put into practice, reliable consolidation of ceramic particles (like cBN or diamond) with refractory metals (such as Ti) is required. Three problems should be solved to sinter ceramic and refractory metals effectively. The first is the poor wettability between ceramic particles and metals, which makes it difficult for sintering. The second is the obvious difference of thermal expansion coefficient between the ceramic particles and refractory metal transition layer with proper thermal expansion coefficient. The third is the weak bonding between the ceramic particles and the metal. Therefore, the sintering with high intensity is difficult. Metallized coating on ceramic particles before sintering can resolve all the three problems. Accordingly, this is a reasonable measure to get an excellent sintering [2].
Deposition techniques involving metallic materials are generally used to improve the surface properties of ceramic materials. To date, techniques such as PVD [3-7], CVD [8], laser process [9,10] and metallic powder sintering [11, 12] have been developed. However, all of these methods are mainly applied on regular surface, such as flat or round planes. For the coated particles, the process complexity increases extensively. In this case, the new method of metallizing needs to be developed [13,14].
The molten salt reaction is a new, simple and low cost method, by which a metallic layer is joined with ceramics very well [15,16]. The deposition of titanium from molten salt systems has been investigated by many researchers. They showed promising results toward the deposition of Ti layer on the surface of several types of ceramic materials such as AlN [17], Al2O3 [18,19], carbon fibers [20], Si3N4 [21] and SiC [22].
In terms of particle coating, the process does not satisfy. Therefore, many efforts are still necessary to find the novel process and materials [23-26]. A number of researchers have reported the formation of thin films of Ti compounds, e.g. Ti metal, TiC and TiN, by disproportionation reactions in molten salts, and valuable anti-corrosion properties, high hardness and good wear resistance have been obtained [27,28]. However, the process is complicated by the formation of the lower valent compounds of titanium (Ti2+, Ti3+), which exist in the molten salts bath as the result of the reactions between metallic titanium and tetra- or tri-valent titanium ions.
In the present work, a molten salt process was used to coat cBN and diamond particles with Ti metal. The produced Ti-coated cBN and diamond particles were subjected to heat-treatment to convert the Ti coating layer to TiN on the surface of cBN and TiC on the surface of diamond particles, and to remove the lower valent titanium compounds which contaminate the coating layers. The produced TiN/cBN and TiC/diamond composite particles were characterized by scanning electron microscope (SEM), differential scanning calorimeter (DSC) and X-ray diffractometer (XRD) in order to evaluate the molten salt coating process of Ti on the surface of the cBN and diamond particles. Additionally, the coating profile of Ti on the particles surfaces was investigated by focused ion beam (FIB) apparatus.
2 Experimental
Diamond and boron nitride powders (particles size ~1 μm) were provided by ILJIN Diamond Co., Ltd., South Korea. The cBN and diamond powders were coated using a molten salt Ti deposition process. Preliminary studies were conducted to optimize the conditions and the Ti molten salt process. Equal amounts of cBN or diamond powders were mixed separately with a molten salt mixture which was composed of 40% KCl, 40% NaCl, and 20% K2TiF6 (mass fraction) (Aldrich Co., Ltd.,) in a medium of 100 mL ethanol to suspend the salts. The mass ratios of cBN to Ti and diamond to Ti were adjusted to be 1:10 in the molten salt reaction.
The aforementioned mixture was baked at 80 °C to evaporate the ethanol and the baking process was completed within 30 min. The dried mixture was blended with sponge Ti powders and spread inside an alumina crucible and subjected to heating in a tube furnace under Ar atmosphere at 900 °C, a flow rate of 0.5 L/min and a heating rate of 10 °C/min for 1 h. After the molten salt reaction was completed, the sample was cooled inside the tube furnace within 8 h. The charge was removed from the furnace and sonicated in a suitable amount of distilled water at 50 °C for 3 h in order to separate the contents from the sponge Ti powders. The produced powders underwent washing with distilled water, filtration and acid treatment with 10% HCl (volume fraction) to dissolve any remaining salts from the molten salt reaction. The produced powders were subsequently washed again with distilled water and dried under vacuum at 80 °C for 2 h. The obtained Ti/cBN and Ti/diamond composite powders were sieved to remove any agglomerated powders. The produced Ti/cBN and Ti/diamond powders were heat-treated separately under H2 atmosphere at 1000 °C for 2 h [18].
The obtained powders were investigated by a RIGAKU D/Max-IIIC (3 kW) XRD diffractometer, SEM and EDAX with a PHILIPS XL30SFEG respectively, in order to evaluate the molten salt coating process of Ti on the surfaces of the cBN and diamond particles. Also, the obtained Ti/cBN particles were investigated by differential scanning calorimeter (DSC) at temperature up to 1400 °C and a rate of 10 °C/min under Ar atmosphere. The morphology and the thickness of the deposited (Ti and TiN) layers on the cBN particles were investigated by a cross-sectional force ion beam (FIB) apparatus.
3 Results and discussion
3.1 Pretreatment of powders
The provided cBN and diamond particles were investigated by SEM in order to characterize their surface morphology. Figures 1(a-c) and 2(a-c) show typical SEM micrographs with low and high magnifications of the investigated cBN and diamond particles, respectively. It was observed that the surfaces of cBN and diamond have smooth morphologies.
The cBN and diamond particles were coated by the Ti molten salt process. The results of preliminary studies on the dissolution of the molten salt reactants indicated that K2TiF6 slightly dissolved in water and ethanol, respectively, whereas it highly dissolved in 10% HCl solution. On the other hand, KCl and NaCl highly dissolved in water, ethanol and 10% HCl, respectively. On the basis of these results, ethanol was selected as a solvent to suspend the cBN and diamond particles and the components of the molten salt reaction. Because ethanol can be evaporated and removed from the powders in a short time (within 30 min), using a magnetic stirrer at 200 r/min and low heating temperature of 80 °C under suction. The cBN and diamond/molten salt reactants capsules consisting of the cBN or diamond particles surrounded by KCl, NaCl and K2TiF6 salts were produced. Coarse sponge Ti particles with 500 μm in size were selected as a source of Ti metal for the molten salt reaction and mixed with the reactant capsules. This process was repeated for each sample of cBN and diamond, respectively.
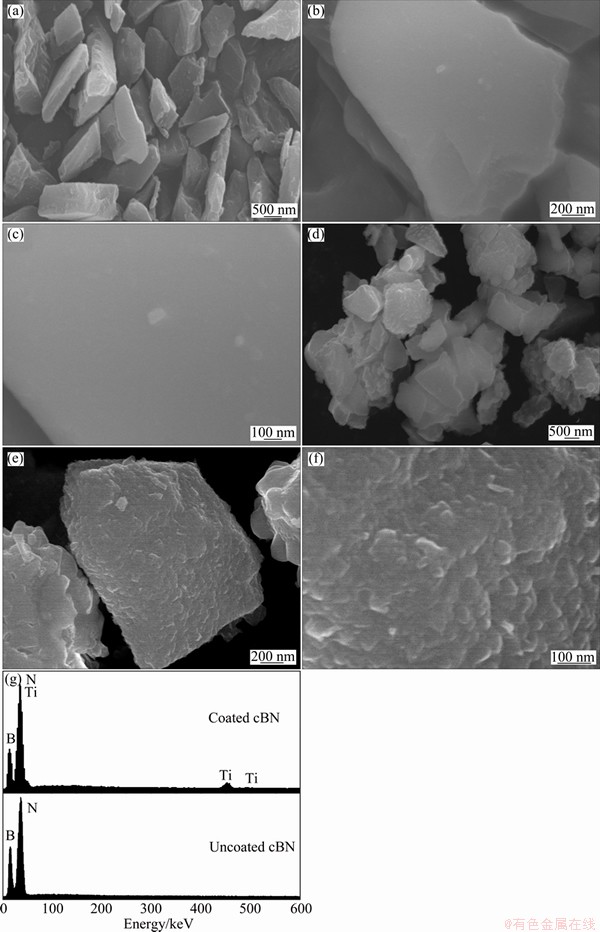
Fig. 1 SEM images with different magnifications for cBN particles (a, b, c) and TiN/cBN coated particles (d, e, f) and EDAX analysis for uncoated and coated cBN particles (g)

Fig. 2 SEM images with different magnifications for diamond particles (a, b, c) and produced TiC/ diamond coated particles (d, e, f) and EDAX analysis for uncoated and coated diamond particles (g)
3.2 Molten salt reaction and microstructure
In the molten salt reaction process, Ti metal was deposited on the surface of cBN and diamond by heating the reactants under Ar atmosphere at 900 °C for 1 h. Figures 1(d-f) and 2(d-f) show SEM images with low and high magnifications for the surface morphologies of the deposited Ti layer on the cBN and diamond particles, respectively. It was observed that after Ti coating via the molten salt method, the cBN and diamond particles surfaces were coated completely with a very fine dense and continuous nano-sized Ti layer. The surface micrograph of the titanium coated particles showed that a continuous and uniform coating layer was successfully obtained. In addition, a fine interfacial structure between the titanium coating and the cBN or diamond substrate was obtained and these layers were converted to the titanium compounds by heat-treatments. The EDAX analysis of the deposited layer in Figs. 1(g) and 2(g) indicated that a uniform layer is composed of TiN in case of cBN particles and TiC layer in case of diamond particles. It was also observed from the results of the EDAX analysis of the coated cBN particles that different kinds of layers were obtained through the molten salt process. Figures 3(a-d) show SEM images with EDAX analysis of the prepared Ti/cBN particles before and after Ti deposition by the molten salt reaction. Figure 3(b) shows the results of an EDAX analysis for the uncoated cBN. Two peaks appear in Fig. 3(b), one for boron and the other for nitrogen. But Fig. 3(c) shows a micrograph of a primary layer composed of network shape connected particles that were aligned on and adhered to the cBN surface. The EDAX analysis for this layer, as shown in Fig. 3(d), reveals a complex chemical composition of Ti, K, Na, F, and O originating from the molten salt reaction mixture, i.e., NaCl, KCl and K2TiF6. Also aluminum peak was observed due to the side reaction between the reactants and the alumina crucible. From the previously mentioned preliminary studies, it was determined that the molten salt complex layer could dissolve in 10% HCl solution within 30 s, and a cleaned cBN/Ti coated powder was obtained. Figure 3(e) shows SEM image of the obtained Ti/cBN particles after surface cleaning. It was observed that the deposited Ti layer had uniform nanostructure morphology. The EDAX analysis of this layer indicates a high intensive peak of Ti and another small peak of the oxygen (Fig. 3(f)). This oxygen peak may be due to oxygen contamination of titanium oxides within the coating layer.
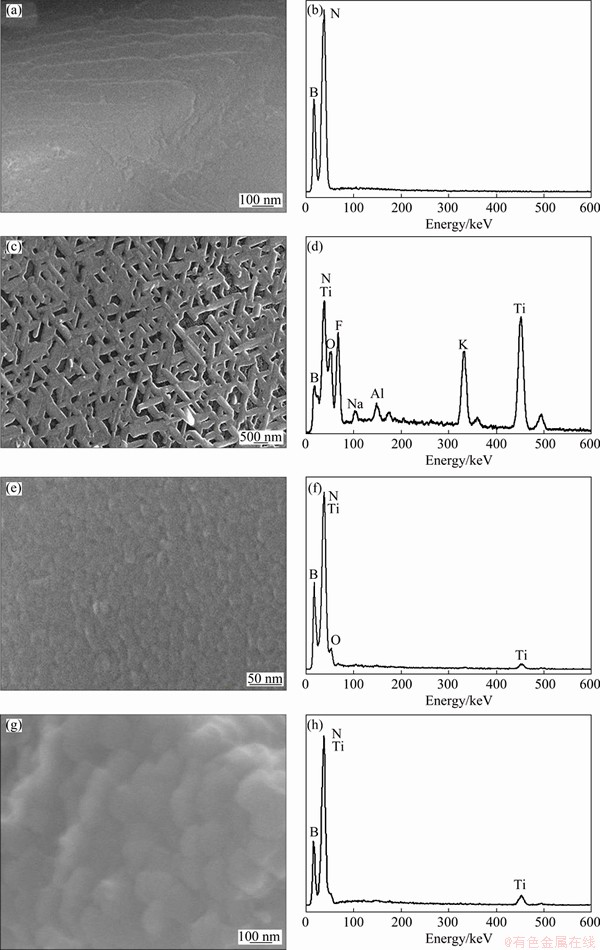
Fig. 3 SEM images and EDAX analysis of cBN particles (a, b), Ti/cBN coated particles before surface cleaning (c, d), Ti/cBN coated particles after surface cleaning (e, f), and heat-treated TiN/cBN coated particles (g, h)
In molten salt reactions at the designed reaction temperature, K2TiF6 dissolved into the NaCl-KCl bath and Ti4+ was produced. The inclusion of K2TiF6 in the molten salt mixture will be advantageous to the formation of titanium cations. Therefore, K2TiF6 was added to the molten salt mixture and the Ti atoms reacted with Ti4+ cations from the decomposed K2TiF6 to produce Ti2+ according to the following chemical equation:
Ti+Ti4+→2Ti2+ (in molten salts) (1)
The stand Gibbs free energy of this reaction is
ΔGΘ =-101.091+0.0116T (kJ/mol)
The coating process began with the attachment of Ti2+ from the molten salt onto the surface of the cBN and diamond particles. This was followed by a disproportionation process on the surface of the cBN and diamond particles whereby Ti2+ was decomposed to produce Ti4+ and Ti as delineated in the following chemical reaction [15-20]:
2Ti2+→Ti+Ti4+ (on cBN or diamond particles) (2)
It was supposed that, in the case of diamond particles, the deposited Ti layer was converted directly to TiC layer due to high reaction affinity between the deposited nano-sized Ti layer on the diamond surface which enhanced the in-situ transformation reaction between the deposited Ti layer and the surface of diamond particles, producing a grey TiC layer as shown in Figs. 2(f) and (g). But in the case of cBN particles, after Ti metallization of the cBN particles, the specimen’s color changed significantly, from original white to silver with metallic luster, due to the deposition of metallic layers on the surface of the cBN particles. This deposited layer of Ti metal on the cBN particles can be converted by heat-treatment at 1000 °C under H2 atmosphere for 2 h to a compound layer composed of TiN as shown in Figs. 3(g) and (h) by the EDAX analysis of the heat-treated layer. The results indicated only one peak for titanium in addition for one peak for boron and another peak for nitrogen. The formation of the compound layer is due to the in-situ transformation reaction between the deposited Ti layer and the surface of the cBN particles under the above controlled conditions according to the following chemical reaction:
3Ti+2BN→TiB2+2TiN (3)
The Ti deposition that occurs in the molten salt bath is a polystep process and the slowest step is the rate determining step. The deposition rate of Ti on cBN or diamond surface at certain temperature indicates that the deposition process may not be controlled by diffusion. It is reasonable to suppose that the disproportionation Reaction (2) may be the rate-determining step of the whole process. Sponge metallic titanium was added to the melt in order to produce Ti3+ needed for the reaction. The addition of Ti0 produces other titanium oxidation state (Reaction (4)) in addition to Reaction (1):
2Ti3++Ti0→3Ti2+ (4)
The presence of Ti2+ in the melt complicates the determination of the actual concentration of Ti3+. The concentration of Ti3+ in the range of the stoichiometric ratio in Reaction (4) is of great importance for establishing a good quality of TiB2 /TiN layer and for sufficient process stability in an industrial application.
The focused ion beam (FIB) cross-sectional morphologies of the prepared Ti/cBN and the related heat-treated specimen are shown in Figs. 4(a) and (b). The coating profile in Fig. 4(a) indicates a bilayer structure of the coating on the surface of the cBN particles. The outer layer is composed of Ti with 350 nm in width and some fine grains of titanium oxide particulates observed from the coating surface contaminate the titanium layer.
The underlying layer is an intermediate layer between cBN and Ti. As anticipated from Reaction (3), this intermediate layer has a chemical composition of TiN with a thickness of 80 nm. This bilayer structure is believed to reflect the two stages during the coating formation. At the threshold of deposition, Ti atoms produced by molten salt reaction aggregated and extensively nucleated on the substrate surface, resulting in the fine coating layer. Then, grains with preferred orientation grew at higher velocity, and constrained the growth of others grains, finally forming the coarse grain layer. However, as a result of heat-treatment, these two layers diffused together to form a single TiN layer with a thickness of 200 nm. The decrease in the thickness of the coating layer by heat-treatment under H2 atmosphere is attributed to the removal of oxide particles from the upper layer (as shown in Fig. 4(a)) and a pure TiN layer with decreasing in the thickness subsequently formed, as shown in Fig. 4(b).
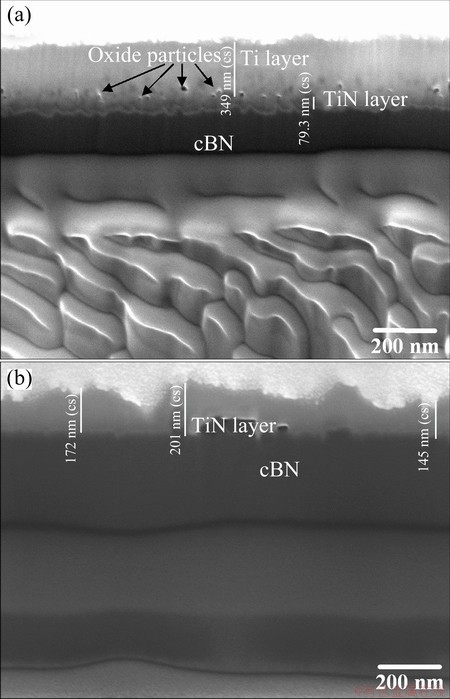
Fig. 4 Focused ion beam surface cross-sectional morphologies for prepared Ti/cBN (a) and heat-treated TiN/cBN coated particles (b)
3.3 DSC and XRD investigations
The results of the DSC analysis shown in Fig. 5 for the obtained Ti/cBN coated particles indicate that there is an inflection of the Cp pattern at 960 °C. It is due to the in-situ reaction between the deposited Ti layer and the nitrogen of the cBN surface, forming a TiN layer on the cBN surface without mass gain or mass loss.
Figure 6 shows XRD patterns of the as-coated Ti/cBN and the heat-treated powders. Four kinds of peaks appear in the XRD pattern of the as-coated Ti/cBN composite powders. The first kind is due to the original cBN, which are the main peaks with the highest intensities; the second kind is due to TiN layer formed by the in-situ reaction between the Ti layer and the nitrogen of the cBN surface; the third is due to the low oxidation state Ti oxides of Ti2O3 included in the coated layer; and the fourth is due to the remained KTiF6 on the cBN surface. Through the above analysis, it can be known that the reduction process consists of several steps: tetravalent titanium in titanium dioxide is first reduced to low valence (Ti3O5 and Ti2O3), and then low valent titanium is reduced to lower valence (Ti3O, Ti2O and TiO) or titanium. However, as a result of heat-treatment of the prepared Ti/cBN under H2 atmosphere at 1000 °C, all these contaminant compounds were removed. Figure 6(b) shows the XRD pattern of TiN/cBN prepared by heat treatment of cBN/Ti composite powders. It was observed that the peaks of Ti2O3 and KTiF4 disappeared, while the intensities of TiN peak increased compared with those of the untreated Ti/cBN coated particles. Due to the formation of TiN layer by heat-treatment, the in-situ reaction between the deposited Ti layer and cBN increased and more TiN formed, as delineated in Reaction (3).
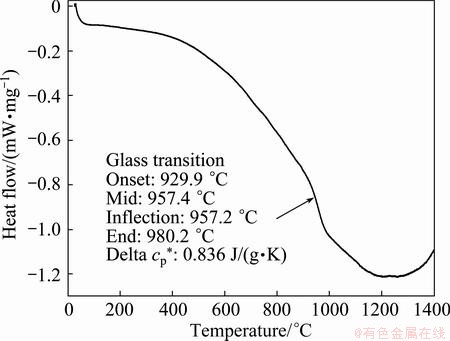
Fig. 5 DSC analysis for Ti/cBN coated particles
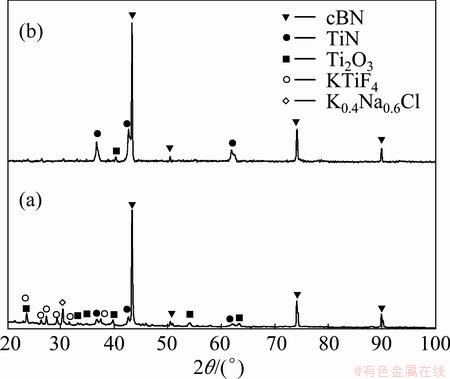
Fig. 6 XRD patterns for prepared Ti/cBN (a) and heat-treated TiN/cBN coated particles (b)
On the other hand, Fig. 7 shows XRD patterns of the uncoated, the as-coated by molten salt process, the acid-treated coated and the as heat-treated TiC/diamond particles. Three peaks appeared in the XRD patterns of all the investigated diamond particles through the preparation steps. But in the case of the as-coated TiC/diamond particles by molten salt process, in addition to four peaks of the diamond particles, another four peaks appeared due to the formation of the TiC layer on the diamond particles, four peaks appeared due to the presence of the remaining KTiF4 (from the molten salt reaction) on the diamond surface and two additional peaks appeared due to the remaining K0.4Na0.6Cl. However, as a result of the acid-treatment, the remaining K0.4Na0.6Cl was removed and the remaining KTiF4 was removed by heat-treatment of the prepared TiC/diamond under H2 atmosphere at 1000 °C.

Fig. 7 XRD patterns for prepared Ti/diamond and heat-treated TiC/diamond coated particles
4 Conclusions
1) Titanium coating can be prepared on the surface of cBN and diamond particles by molten salt reaction. The metallized coating is dense and homogenous. The coating seals the pores on the surface of the particles and covers the particle surface area completely.
2) The main layer of the metallized coating is a uniform Ti layer. The interfaces of cBN and metallized coating are TiN in the case of cBN and TiC in the case of diamond particles, and can be converted completely by the in-situ transformation of Ti under proper atmosphere to TiN in the case of cBN and to TiC layer in the case of diamond particles by heat treatments.
3) The ceramic coating layers (TiN or TiC) can be adhered well with the surface of cBN or diamond particles, and enhance the consolidation of the particles, which will improve the performance of the sintering process of the cBN as well as the diamond particles under moderate sintering conditions.
References
[1] PARK H S, RYOO M H, DAOUSH W M, HONG S H. High hardness coating powder and preparation method thereof: WIPO patent, 2010056077 [P]. 2010-05-20.
[2] NASLAIN R. Design, preparation and properties of non-oxide CMCs for application in engines and nuclear reactors: An overview [J]. Composites Science and Technology, 2004, 64: 155-170.
[3] RONG C, ZHANG J Z, LIU C Z, YANG S Z. Surface metallization of alumina ceramics by pulsed high energy density plasma process [J]. Applied Surface Science, 2002, 200: 104-110.
[4] PIEKOSZEWSKI J, KRAJEWSKI A, PROKERT F, SENKARA J, STANISLAWSKI J, WALIS L, WERNER Z, WLOSINSKI W. Brazing of alumina ceramics modified by pulsed plasma beams combined with arc PVD treatment [J]. Vacuum, 2003, 70: 307-312.
[5] WARREN J, ELZEY D M, WARREN H G. A fiber damage model forearly stage consolidation of metal-coated fibers [J]. Acta Materialia, 1997, 45: 973-986.
[6] HAN H W, LEE N E. Sputter deposition modeling of Ti thin film on a sharp tip [J]. Thin Solid Films, 2005, 475: 144-149.
[7] LI X, JIN Y, LIU D, ZENG X, YANG R. Topographical evolution of magnetron sputtering Ti thin films during oxidation observed by AFM [J]. Surface Review and Letters, 2011, 18: 61-69.
[8] LAIMER J, FINK M, MITTERER C, STORI H. Plasma CVD of alumina—Unsolved problems [J]. Vacuum, 2005, 80: 141-145.
[9] DESILVA M J, PEDRAZA A J, LOWNDES D H. Electroless copper films deposited onto laser-activated aluminum nitride and alumina [J]. Journal of Material Research, 1994, 9: 1019-1027.
[10] MENECIER S, JARRIGE J, LABBE J C, LEFORT P. Identification of parameters involved in the bonding of copper tracks on alumina substrates by a laser process [J]. Journal of European Ceramic Society, 2007, 27: 851-854.
[11] MOUSTAFA S F, RASHAD W M, EL-SHEREAFY E E. Cu/matrix composites produced with either coated or uncoated reinforcement powders [J]. Canadian Metallurgical Quarterly, 2001, 40: 533-536.
[12] DAOUSH W, BRADBURY W, OLEVSKY E, GERMAN R M. Consolidation of Si3N4/Cu composite powders fabricated by electroless deposition technique [C]//Proceedings of Composite Materials (ICCM18). Jeju, Korea, 2011: 1-5.
[13] MANDAL S, RAO V, RAY A K. Characterization of the brazed joint interface between Al2O3 and (Ag-Cu-Ti) [J]. Journal of Material Science, 2004, 39: 5587-5590.
[14] VOLCEANOV E, VOLCEANOV A, STOLERIU T. Influence of sintering environment on zirconia–metal carbides characteristics [J]. Journal of European Ceramic Society, 2007, 27: 759-762.
[15] POLYAKOV L P, STANGRIT P T, POLYAKOV E G. Electrochemical study of titanium in chloride-fluoride melts [J]. Electrochimica Acta, 1986, 31: 159-161.
[16] SEN C G, OKIDO M, OKI T. Electrochemical studies of titanium in fluoride-chloride molten salts [J]. Journal of Applied Electrochemistry, 1988, 18: 80-85.
[17] LI J, WEI P, QILIANG H, CHEN J, ZHANG Z. Mechanism of titanium deposition on AlN surface by molten salt reaction [J] Materials Letters, 2003, 57: 1369-1373.
[18] BAUMLI P, SYTCHEV J,  Z H, KAPTAY G. Interaction between a titanium-containing molten salt and an alumina plate [J]. Materials Science Forum, 2005, 473-474: 39-44.
Z H, KAPTAY G. Interaction between a titanium-containing molten salt and an alumina plate [J]. Materials Science Forum, 2005, 473-474: 39-44.
[19] LI J, WEI P, YUAN Z, CHEN Y. Titanium metallization of alumina ceramics by molten salt reaction [J]. Applied Surface Science, 2008, 254: 4584-4590.
[20] LI X, DONG Z, WESTWOOD A, BROWN A, ZHANG S, BRYDSON R, LA N, RAND B. Preparation of a titanium carbide coating on carbon fiber using a molten salt method [J]. Carbon, 2008, 46: 305-309.
[21] WEI P, LI J, CHEN J. Titanium metallization of Si3N4 ceramics by molten salt reaction: Coating microstructure and brazing property [J]. Thin Solid Films, 2002, 422: 126-129.
[22] WENYUAN Z, XIAOHUI Z, YONGGUO D, WEIJUN Z, YUFENG L. Ti metallization of Cf/SiC composites surface by molten salt reaction [J]. Rare Metal Materials and Engineering, 2009, 38: 209-0213.
[23] LIU Y, ZHU Y, YANG Y, LIU X. Microstructure of reaction layer and its effect on the joining strength of SiC/SiC joints brazed using Ag-Cu-In-Ti alloy [J]. Advanced Ceramics, 2014, 3: 71-75.
[24] LI J. Ti metallization of AlN surface by molten salt reaction [J]. Journal of Inorganic Materials, 2003, 18: 1086-1090.
[25] LIANG H, WEI P, ZHONG H. A study of AlN ceramic surface titanium metallization by molten salt disproportional reactions [J]. Materials Review, 2002, 16: 66-68.
[26] DUDA C. Microstructural characterization of liquid route processed Ti 6242 coating of SCS-6 filaments [J]. Composites Part A: Applied Science and Manufacturing, 2004, 35: 511-517.
[27] YOSHIDAA H, KUMEA S. Very high pressure sintering of cBN fine particles coated with TiN-TiB2 layer formed by disproportionation reaction in molten salts [J]. Journal of Materials Research, 1997, 12: 585-588.
[28] SEN C, OKIDO M, OKI T. Electrochemical studies of titanium ions (Ti4+) in equimolar KCl-NaCl molten salts with 1 wt.% K2TiF6 [J]. Electrochemica Acta, 1987, 32: 1637-1642.
Walid M. DAOUSH1, Hee S. PARK2, Soon H. HONG3
1. Department of Production Technology, Faculty of Industrial Education, Helwan University, Cairo 11511-11668, Egypt;
2. Institute of Industrial Technology, ILJIN Diamond Co., Ltd., Chungcheongbuk 369-824, Korea;
3. Department of Materials Science and Engineering, Korea Advanced Institute of Science and Technology, Daejon 305-701, Korea
摘 要:用钛熔盐沉积及热处理工艺分别制备碳化钛涂覆的立方碳化硼颗粒(TiN/cBN)及碳化钛涂覆的金刚石颗粒(TiC/金刚石)。将cBN或金刚石颗粒分别与钛粉和KCl、NaCl和K2TiF6熔盐混合。将所得混合物在Ar气氛中加热至900 °C,然后在H2气氛中于1000 °C进行热处理。采用扫描电镜、X射线衍射和聚焦离子束技术对所制得颗粒进行表征。结果表明:cBN和金刚石颗粒表面已覆盖了纳米钛层。对Ti/cBN和TiC/金刚石涂层颗粒进行热处理后,颗粒表面沉积的Ti层与cBN和金刚石颗粒发生了原位化学反应,分别转化为钛化合物TiN和TiC。
关键词:熔盐反应;钛;沉积;立方氮化硼;金刚石;涂层;TiN;TiC
(Edited by Wei-ping CHEN)
Corresponding author: Walid M. DAOUSH; E-mail: waliddaoush@techedu.helwan.edu.eg
DOI: 10.1016/S1003-6326(14)63502-0

