Removal of copper from nickel anode electrolyte through ion exchange
来源期刊:中国有色金属学报(英文版)2009年第1期
论文作者:陈爱良 邱冠周 赵中伟 孙培梅 余润兰
文章页码:253 - 258
Key words:nickel anode electrolyte; copper removal; cupric deoxidization; anion ion exchange; breakthrough capacity
Abstract: An novel method for removal of copper from nickel anodic electrolyte through ion exchange was studied after cupric deoxidization. Orthogonal design experiments show the optimum conditions of deoxidizing cupric into Cu+ in the nickel electrolyte are the reductive agent dosage is 4.5 times as the theoretic dosage and reaction time is 0.5 h at 40 ℃ and pH 2.0. Ion exchange experiments show that the breakthrough capacity(Y) decreases with the increase of the linear flow rate(X): Y=1.559-0.194X+ 0.006 7X2. Breakthrough capacity increases with the increase of the ratio of height to radius(RRH). The higher the initial copper concentration, the less the breakthrough capacity(BC). SO42- and nickel concentration have no obvious change during the process of sorption, so it is not necessary to worry about the loss of nickel during the sorption process. Desorption experiments show that copper desorption from the resin is made perfectly with NaCl solution added with 4% (volume fraction) H2O2 (30%) and more than 100 g/L CuCl2 solution is achieved.

CHEN Ai-liang(陈爱良)1, 2, QIU Guan-zhou(邱冠周)1, ZHAO Zhong-wei(赵中伟)2,
SUN Pei-mei(孙培梅)2, YU Run-lan(余润兰)1
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2.School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 25 February 2008; accepted 4 July 2008
Abstract: An novel method for removal of copper from nickel anodic electrolyte through ion exchange was studied after cupric deoxidization. Orthogonal design experiments show the optimum conditions of deoxidizing cupric into Cu+ in the nickel electrolyte are the reductive agent dosage is 4.5 times as the theoretic dosage and reaction time is 0.5 h at 40 ℃ and pH 2.0. Ion exchange experiments show that the breakthrough capacity(Y) decreases with the increase of the linear flow rate(X): Y=1.559-0.194X+ 0.006 7X2. Breakthrough capacity increases with the increase of the ratio of height to radius(RRH). The higher the initial copper concentration, the less the breakthrough capacity(BC). SO42- and nickel concentration have no obvious change during the process of sorption, so it is not necessary to worry about the loss of nickel during the sorption process. Desorption experiments show that copper desorption from the resin is made perfectly with NaCl solution added with 4% (volume fraction) H2O2 (30%) and more than 100 g/L CuCl2 solution is achieved.
Key words: nickel anode electrolyte; copper removal; cupric deoxidization; anion ion exchange; breakthrough capacity
1 Introduction
The concentrations of other heavy metals must be reduced to very low levels prior to nickel electro- deposition in the anode nickel electrolyte because of the effect on the product purity of electrodeposited nickel. Nowadays other heavy metals except copper can be kept at the low levels satisfied with the product purity of electrodeposited nickel. However, it is difficult to separate copper from nickel solution due to the certain similar chemical properties[1-3], and how to solve this problem has become a vital part of the national important projects of the Eighth and Ninth “Five Year Plan”.
Many researches for copper treatment have been conducted. Precipitation has been widely used to separate copper from nickel electrolyte based on the different characters of copper and nickel, and the method has been used widely for its simplicity[4-6]. Especially for sulfide precipitation process, it has better selectivity because copper has strong affinity for sulfur. So much research has been carried on to remove copper from anode nickel electrolyte with many substances[7-11], such as sulfureted hydrogen, sodium sulfide, active nickel sulfide, “NSH” reagent, sulphur and sulfur dioxide, active anode mud and sodium and nickel thiosulfate. Although these sulphides may bring about high reaction rate with simple manipulation, too much residue or sludge was produced. However, there are many valuable metals in the residue including nickel, copper, and even noble metals, which are difficult to be recovered[12-14]. Moreover, there existed large amount of residue with some toxic compounds and it could pollute environment [15-16].
Now ion exchange is often used as an effective method to extract valuable metals from water solution in the hydrometallurgy. And the anion exchange method in the hydrochloric acid solution has been applied for purification of Co, and the good results for separation of metallic impurities from cobalt chloride have been reported[17]. Therefore, we may try to remove copper from anode nickel electrolyte with ion exchange. However, copper and nickel mainly exist in the form of Cu2+ and Ni2+ in the solution regardless of their complexes with chlorine. So it is difficult to separate copper with cation resin exchange. And it cannot be satisfied with demands of production if anion resin exchange is simply used for copper treatment. The anode nickel electrolyte must be dealt with before anion resin exchange is used.
In this work, a reductive agent was added to reduce Cu(Ⅱ) into Cu(Ⅰ), and Cu(Ⅰ) can become anion complexes with chlorine but it is difficult for nickel in the nickel electrolyte at [Cl-]=70-80 g/L. So nickel and Cu(Ⅰ) can be separated with anion exchange.
2 Experimental
2.1 Raw materials
The chemical composition of nickel electrolyte is very complex and the components used in a nonferrous metal company are listed in Table 1. The concentration of nickel and chlorine is far greater than that of copper. It can be seen that Me2+-Cl- complexes are formed easily due to the high concentration of chlorine ions. Although the concentration of SO42- is high and is greater than that of Cl-, it is difficult to form complexes with copper and nickel. The concentration of H3BO3 is too low to be considered in the solution at pH 4.5.
Table 1 Components of nickel electrolysis solution
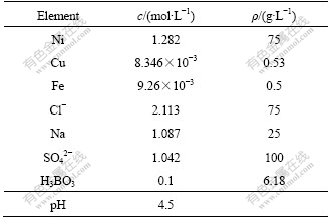
2.2 Experimental methods
The initial nickel electrolyte solutions were placed in a 500 mL cell using a water bath circulator and were regulated at a certain pH with diluted NaOH or HCl. And then sodium sulfite was added and Cu2+ was reduced to Cu+. Finally, the solutions were poured into the column with 717# strongly basic anion exchange resin and copper could be absorbed into resin from the dealt nickel electrolyte solution. When the copper concentration of the outflow from the column arrived 3×10-6 mol/L, the sorption must stop and the sorption capacity was defined as breakthrough capacity(BC). Breakthrough capacity in mmol/g dry resin was calculated.
![]()
where c0 is the initial Cu concentration, ca is the average Cu concentration of the outflow, V is the volume of the outflow, MCu is the molar mass of copper, and mrd is the mass of dry resin.
After sorption, washing the remnants in the column was needed and then desorption was made by NaCl solution with a small amount of H2O2 at low pH. Copper went into solution in the form of CuCl2 from resin. The resin can be regenerated and may be reused.
The copper concentration of the outflow solution was analyzed with an atom absorption spectrophotometer AAS 1 N (Zeiss Jena, Germany).
2.3 Reagents
Ion exchange resin was 201×7 strongly basic anion exchange resin in chloride form. 99.98% sodium sulfite, initial nickel electrolyte solution from a company in China and deionized water were used to prepare the required solutions.
3 Results and discussion
3.1 Deoxidization
3.1.1 Deoxidization of cupric
During the deoxidization of cupric from solution, it is found that different end points of potential(EPP) will lead to different breakthrough capacity, as shown in Fig.1.
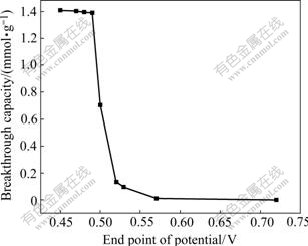
Fig.1 Effect of end point of potential on breakthrough capacity
Fig.1 shows that breakthrough capacity decreases with the increase of the end point of potential. When the end point of reductive potential is between 0.52 V and 0.49 V, breakthrough capacity increases by 10 times and arrives 1.39 mmol/g dry resin at 0.49 V from 0.14 mmol/g dry resin at 0.52 V. When EPP is lower than 0.49 V, BC increases slowly and it is only 1.41 mmol/g dry resin at 0.45 V. When the end point of potential arrives 0.72 V, there is almost nothing to exchange. With the development of cupric deoxidization, the lower the potential, the more the breakthrough capacity is. This is because that when sodium sulfite is added into the solution, cupric may be deoxidized into Cu+ as follows:
2Cu2++SO32-+H2O=2Cu++SO42-+2H+ (1)
Cu2+, Cu+ and Ni2+ ions can combine complexes with chlorine as follows:
Cu2++iCl-=CuCli2-i (i=1, 2, 3, 4) (2)
Cu++iCl-=CuCui1-i (i=1, 2, 3) (3)
Ni2++iCl-=NiCli2-i (i=1, 2, 3, 4) (4)
The potential of the initial solution is 0.72 V, indicating that it is difficult for cupric to form anion complexes. It is also difficult for Ni2+ to combine anion complexes with chlorine[16-17] according to the stable constants of complex from Table 2. But it is easy for the Cu+ to form anion complexes with chlorine. Especially, anion complexes of CuCl2- and CuCl32- are more easily formed than other ions mentioned above because lgβ2 and lgβ3 of Cu+ are 6.06 and 6.89, respectively. Moreover, the stable constant of Cu+ is greater than that of Cu2+. However, cupric exists in the form of CuCl+ cation complexes. It is confirmed that cupric must be turned into Cu+ so as to form anion complexes.
Table 2 Stable constants of copper and nickel complex
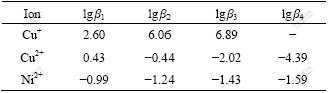
Thus, the end point of reductive potential has great effects on the breakthrough capacity of resin. During the process of deoxidization, the less the potential in the solution, the more the CuCli(i-1)- is produced and the easier the anion exchange.
3.1.2 Orthogonal design experiment
In order to confirm the deoxidization conditions of cupric, factorial design experiment is needed. Some factors might have great effects on the deoxidization, such as reductive agent dosage, pH of solution, time and temperature of reaction. Now orthogonal experiments were designed with the four factors and three levels with the aim value of EPP as listed in Table 3.
Table 3 displays the effect sequence of the above four factors: A>B>C>D. That is to say, the reductive agent dosage has the greatest effect on the reductive potential of cupric, pH of solution and reaction time have less effect, and reaction temperature has the least effect. It is also shown that the optimum conditions of the reductive cupric to Cu+ in the nickel electrolyte are: the reductive agent dosage is 4.5 times as the theoretic dosage and reaction time is 0.5 h at 40 ℃ and pH 2.0.
3.1.3 Effect of reductive agent dosage
The effect of reductive agent dosage on the end point of reductive potential is shown in Fig.2. It can be seen that EPP decreases with the increase of reductive agent dosage. The more the reductive agent dosage, the lower the end point of potential is.
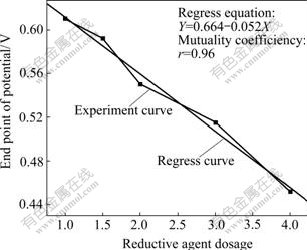
Fig.2 Effect of reductive agent dosage on end point of reductive potential
3.2 Sorption
Under the above optimum reductive conditions, cupric was deoxidized to Cu+ in the nickel electrolyte solution at the stirring rate of 400 r/min, and then the single factor test for the anion-exchange of the deoxidized solution was applied to optimize the conditions for removing copper.
The process of Cu+-Cl- anion complexes sorption on 201×7 strongly basic anion exchange resin can be presented by
![]() (5)
(5)
Cu+-Cl- anion complexes replace chlorine of the resin and copper enters into the resin. Thus, copper is removed from the nickel electrolyte solution.
3.2.1. Effect of sorption speed on copper sorption
Copper sorption speed may be defined by linear flow rate, which can decide the breakthrough capacity, as shown in Fig.3.
It can be seen from Fig.3 that the breakthrough capacity decreases with the increase of the linear flow rate. Complexes have no time to replace chlorine of the resin with too high linear flow rate. The more the linear flow rate, the less the time for copper to exchange with resin is, and the less the breakthrough capacity is. It is harmful for copper sorption with too high linear flow rate. But it will reduce the production efficiency with too slow linear flow rate. A suitable rate is needed. There is a relationship between the linear flow rate and the breakthrough capacity by fitting as shown in Fig.3.
Table 3 Result of factorial design experiments
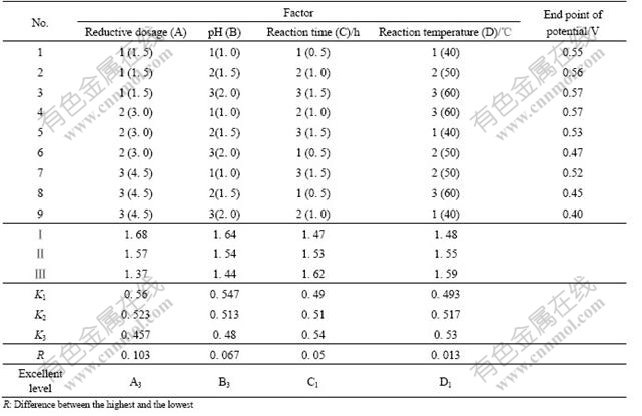
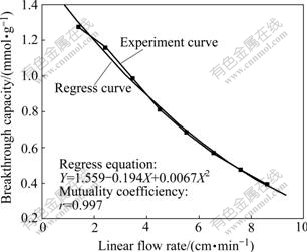
Fig.3 Effect of linear flow rate on breakthrough capacity
3.2.2. Effect of height to radius(RRH) ratio on copper sorption
In order to improve removal copper productivity, it is necessary to check the effect of RRH of resin exchanger column. Anion exchange experiments were done on condition that RRH was changed at the linear flow rate of 5 cm/min. The relationship among the mass of dry resin (mr), amount of substance of copper (nCu) and breakthrough capacity is listed in Table 4.
Table 4 Effect of ratio of height to radius on breakthrough capacity
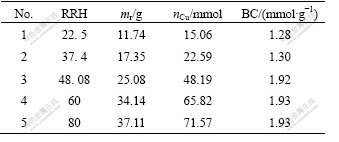
From Table 4, we can see that the ratio of breakthrough capacity increases with the increase of RRH. When RRH reaches 48.08, the breakthrough capacity has light change. So the optimum value of RRH might be considered to be more than 48.08, but it is easy for resin to be crushed with so high RRH. Fortunately, it can be solved by the way that resin exchanger column might be jointed in tandem.
3.2.3 Effect of initial copper concentration on break- through capacity
When the initial copper concentrations are changed at resin volume of 39.25 mL, the resin breakthrough capacities are shown in Fig.4. It can be seen that resin breakthrough capacities decrease with the increase of initial copper concentrations. That is to say, the higher the initial copper concentration, the more difficult for the resin to absorb copper from solution under the stable linear flow rate.
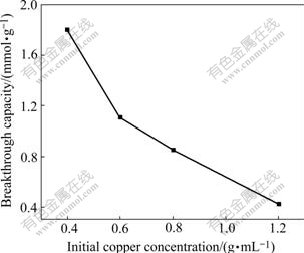
Fig.4 Effect of initial copper concentration on breakthrough capacity
3.2.4 Selectivity sorption of other ions
There are other ions with high concentration in the electrolyte solution except CuCl2- and CuCl32-. Before sorption, the initial SO42- and nickel concentrations were 80 and 75 g/L, respectively. It is necessary to check the concentration change of SO42- and nickel after sorption. Fig.5 shows that SO42- and nickel concentrations in the outflow decrease only by 0.5 g/L with the increase of outflow volume during the process of sorption. This shows that there is no obvious change in the concentration of SO42- and nickel during the process of copper sorption. So it is not necessary to worry about the loss of nickel during the sorption process.
3.3 Desorption
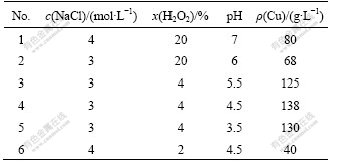
Copper desorption from the resin was made perfectly by 3 mol/L NaCl solution with 4% (volume fraction) H2O2 (30%) at the desorption rate of 10 cm/min when pH=4.5-5.5. Copper went into solution in the form of CuCl2 from resin with copper and more than 100 g/L CuCl2 solution was achieved, which could be used to produce copper salt or electrolyte copper as listed in Table 5. The concentration of NaCl has little effect on the CuCl2 concentration when it is between 3.0 and 4.0 mol/L. The CuCl2 concentration is affected greatly by the H2O2 concentration. If H2O2 concentration is not enough, some Cu+ ions can not oxidate into Cu2+ ions and become CuCl deposition. CuCl deposition will cover the surface of the resin and block the desorption. Only when CuCl deposition is oxidated by oxidant like H2O2 into CuCl2, could copper enter into water in the form of ions. However, too much H2O2 (20% volume fraction) will damage resin and it is not helpful for production. According to the reaction (6), pH has a great effect on the desorption because H+ is consumed in the process. Reaction (6) goes on rightward at lower pH and copper is desorpted fully. When CuCl(s) deposition is oxidated into soluble CuCl2, the reaction (6) goes on rightwards and copper desorption is perfectly accomplished. So, only when solution is acidic and pH is less than 6, can CuCl be oxidated adequately by H2O2 into soluble CuCl2 with more than 100 g/L.
![]()
![]() (6)
(6)
Moreover, the resin can be regenerated after washing and may be reused.

Fig.5 Change of SO42- and nickel concentration with outflow volume
Table 5 Copper concentration in solution after desorption
4 Conclusions
1) Orthogonal design experiments show that the effect sequence of the four factors is reductive dosage>pH>reaction time>reaction temperature. This shows that the optimum conditions of the reductive cupric to Cu+ in the nickel electrolyte are: the reductive agent dosage is 4.5 times as the theoretic dosage and reaction time is 0.5 h at 40 ℃ and pH 2.0.
2) It is found that the reductive potential has a great effect on the resin breakthrough capacity during the sorption experiments. Resin breakthrough capacity increases with the decrease of reductive potential. When the potential is between 0.47 V and 0.45 V, copper breakthrough capacity is the greatest.
3) Ion exchange experiments show that the break- through capacity decreases with the increase of the linear flow rate in the way of conic by fitting:
Y=1.559-0.194X+0.006 7X2
4) Breakthrough capacity increases with the increase of the ratio of height to radius. When the ratio reaches 48.08, the breakthrough capacity has little change. The higher the initial copper concentration, the less the breakthrough capacity is. SO42- and nickel concentration will have little change with the increase of outflow volume during the process of sorption.
5) Desorption experiments show that copper desorption from the resin is made perfectly by 3 mol/L NaCl solution with 4% (volume fraction) H2O2 (30%) at the desorption rate of 10 cm/min when pH is 4.5-5.5. Copper goes into solution in the form of CuCl2 from resin with copper and more than 100 g/L CuCl2 solution is achieved.
References
[1] REN Hong-jiu, WANG Li-chuang. Hydrometallurgy handbook of nonferrous metal abstract (copper and nickel volume) [M]. Beijing: Metallurgy Industry Press, 2000. (in Chinese)
[2] LI Hong-gui. Metallurgy principium [M]. Beijing: Science Press, 2005: 185. (in Chinese)
[3] LEE C I, YANG Wan-fa, HSIEH C I. Removal of copper (II) by manganese-coated sand in a liquid fluidized-bed reactor [J]. Journal of Hazardous Materials, 2004, 5(114): 45-51.
[4] PENG Rong-qiu. Nickel metallurgy [M]. Changsha: Central South University Press, 2004: 133-134. (in Chinese)
[5] LIU Jian-she, SHU Yu-de, CHEN Bai-zhen, YAN Xiao-xiang. Remove of copper from anode solution in nickel electrolyte [J]. Journal of Central South University of Technology: Natural Science, 1995, 26(1): 56-60. (in Chinese)
[6] GAMBURG Y D, GROSHEVA M Y, BIALLOZOR S. The electrochemical deposition of nickel from electrolytes containing malonic acid [J]. Surface and Coatings Technology, 2002, 150(1): 95-100.
[7] SINGH V, PANDEY P. Electrodeposition of nickel composites from water-diethanolamine bath [J]. Surface and Coatings Technology, 2006, 200(15): 4511-4514.
[8] LIRA-CANT? M, SABIO A M, BRUSTENGA A. Electrochemical deposition of black nickel solar sorption coatings on stainless steel AISI316L for thermal solar cells [J]. Solar Energy Materials and Solar Cells, 2005, 87(6): 685-694.
[9] GEOFFROY R P, EVERALDO C. Electrodeposition of nickel on carbon felt [J]. Electrochimica Acta, 2004, 49(27): 4933-4938.
[10] SAMUEL B E, JENNIFER L H, ROY D. Voltammetric and amperometric analyses of electrochemical nucleation: Electro- deposition of copper on nickel and tantalum [J]. Journal of Electroanalytical Chemistry, 2004, 568(2): 121-133.
[11] GONG Zhu-qing, HUANG Jian, JIANG Han-ying. Study of the comprehensive retrieve utilize and the treatment of mine acid wastewater [J]. Journal of Central South University of Technology: Natural Science, 1996, 27(4): 432-435. (in Chinese)
[12] LAZARIDIS N K, PELEKA E N, MATIS K A. Copper removal from effluents by various separation techniques [J]. Hydrometallurgy, 2004, 74(1): 149-156.
[13] RAO K A, NATARAJAN R, PADMANABHAN N P H. Studies on recovery of copper, nickel, cobalt and molybdenum values from a bulk sulphide concentrate of an Indian uranium ore [J]. Hydrometallurgy, 2001, 62(2): 115-124.
[14] RESENDE P C, BARRADO F S, MARTINS A H. Sulfuric activation of a brazilian manganese ore for heavy metals removal [J]. Hydrometallurgy, 1999, 3(51): 325-333.
[15] MEI Guang-gui, ZHONG Yun-bo, ZHONG Zhu-qian. The thermodynamic analysis of purifying spent copper electrolyte using sulfide precipitation method [J]. Journal of Central South University of Technology: Natural Science, 1996, 27(1): 31-35. (in Chinese)
[16] MCGREGOR R G, BLOWES D W. The physical, chemical and mineralogical properties of three cemented layers within sulfide-bearing mine tailings [J]. Journal of Geochemical Exploration, 2002, 76(3): 195-207.
[17] DAI Z R, BRADLREY J P. Iron-nickel sulphides in anhydrous interplanetary dust particles [J]. Geochimica et Cosmochimica Acta, 2001, 49(20): 3601-3612.
Foundation item: Project supported by the Postdoctoral Foundation of Central South University and Minerals Processing and Bioengineering, China
Corresponding author: CHEN Ai-liang; Tel: +86-731-8830476; Fax: +86-731-8830477; E-mail: chenailiang@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(08)60261-7

