文章编号:1004-0609(2007)03-0487-05
Cd2+-H2O系羟合配离子配位平衡
柴立元,常 皓,王云燕,舒余德,李 敬,袁 林,王 璞,方 艳,赵 堃
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:根据配位化学热力学平衡原理,绘制了Cd2+-H2O系配合离子浓度pc—pH图、镉羟合配离子分率αn—pH图及Cd(OH)2条件溶度积pKS—pH图。pc—pH图描述了Cd(OH)2(s)溶解平衡时,镉的总离子平衡浓度与pH的关系。当pH为9.84~13.31时,Cd(OH)2的溶解度最小;αn—pH图指出了各种羟合配离子分率与pH关系,每种羟合配离子都对应有其存在的最佳pH范围。Cd(OH)2(s)的条件溶度积pKS—pH图表明:当pH值在9.5~10.5范围内,Cd(OH)2(s)的条件溶度积最小。研究结果可为中和水解法去除废水中镉等技术提供理论依据。
关键词:Cd2+-H2O系;含镉废水;配位离子;pc—pH图;条件溶度积;配位离子分率
中图分类号:O 641.4 文献标识码:A
Equilibrium of hydroxyl complex ions in Cd2+-H2O system CHAI Li-yuan, CHANG Hao, WANG Yun-yan, SHU Yu-de, LI Jing,
YUAN Lin, WANG Pu, FANG Yan, ZHAO Kun
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The diagrams for the concentration of complex ions pc—pH, the ratio of cadmium hydroxy complex ions αn—pH and the conditional solubility product of Cd(OH)2 to pH value in Cd2+-H2O system were drawn, respectively. The relationship between the equilibrium concentration of total cadmium ions and pH value was shown in Cd2+-H2O system, when the dissolution of Cd(OH)2 is in equilibrium. The solubility of Cd(OH)2 is the least at pH value of 9.84-13.31. The diagram for pKS—pH shows that each hydroxy complex ion existing in this system is dependent upon an optimized pH value. The diagram for the conditional solubility product pKS—pH indicates that the pKS reaches the minimum value at pH value ranging from 9.5 to 10.5. The results can provide a theoretical basis for the technology on removal of cadmium ion from the wastewater by neutralized hydrolyzation method.
Key words: Cd2+-H2O system; cadmium wastewater; complex ions; pc—pH diagram; conditional solubility product; complex ions ratio
有色冶金及电镀工业常产生大量含镉重金属废水,对环境造成严重污染,各行业必须对含镉废水进行净化处理。目前主要处理方法采用中和水解法[1]。为了对中和法工艺提供理论依据,有研究曾对镉离子的水解行为进行了热力学分析,但是没有系统考虑水溶液体系中金属离子的所有羟合配离子[2];或者局限在单一配位体上进行分析[3-6]。因此,所得结论势必会不完善、不全面[7-8],影响对工艺的正确指导。
本文作者在全面考虑镉在水中存在的各种羟合配离子的基础上引入配位化学[9]和水化学[10]的有关概念,对金属离子Cd2+-H2O体系中羟合配离子的热力学平衡进行详细全面的分析研究,绘制体系的配合离子浓度pc—pH图、镉羟合配离子分率αn—pH图及Cd(OH)2条件溶度积pKS—pH图。试图用这些热力学图揭示镉离子在水溶液中的存在形态及Cd(OH)2的溶解度随pH值的变化规律,从热力学角度确定Cd(OH)2在水溶液中的最小溶解度,及镉离子从电解液和废水中脱除的最佳pH值的范围。这些热力学图在中和水解法中的应用比电位-pH图[11-12]更具实用价值,能为中和水解法净化含镉废水及湿法冶金除杂质镉提供更加严格的理论依据。
1 pH值对Cd2+离子羟基配位平衡的影响
含镉水溶液中,主要有CdOH+、Cd(OH)2、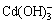 和
和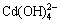 4种配离子存在,在298.15 K条件下逐级稳定常数[13]分别为:
4种配离子存在,在298.15 K条件下逐级稳定常数[13]分别为:

将式(10)~(14)在pc—pH坐标系中作图(见图1)[15]。

图1 298.15 K时Cd2+-H2O系pc—pH图
Fig.1 Diagram of pc—pH of Cd2+-H2O system at 298.15 K
图中每一根直线表示与Cd(OH)2固相平衡时对应的配位离子浓度与pH值的关系,所有直线包围的面积是Cd(OH)2沉淀区域(阴影部分面积),即Cd(OH)2固相的稳定区。其它区域为镉离子非饱和区。组成此稳定区的边界线近似地表示Cd2+-H2O系中Cd2+的总溶解度与pH值的关系。由图1可看出,当pH值为9.84~13.31时,镉的溶解度最小,为0.601 mg/L;当pH<9.84及pH>13.31时,镉的溶解度都会增加。
2 pH值对Cd2+离子形成羟合配离子形态的影响
在水溶液中,重金属Cd2+离子会形成配离子CdOH+、Cd(OH)2、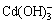 、
、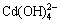 。有关配位反应及逐级累积常数[13]分别为:
。有关配位反应及逐级累积常数[13]分别为:

从上述关系式可以看出,各种离子的浓度分率αn与pH值([OH-])有很大的关系。将不同的pH值代入式(33)可以得到相应pH值时的α0。再应用式(29)~(32)可以求出α1、α2、α3和α4。以αn为纵坐标,pH值为横坐标作图,得图2。
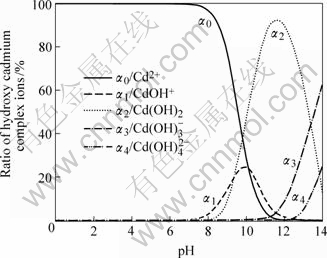
图2 镉羟合配离子分率αn—pH图
Fig.2 αn—pH diagram of ratio of cadmium hydroxy complex ions
由图2可看出,在不同pH值时,金属镉离子以不同羟合配离子存在,当pH值小于9.8时,主要以游离Cd2+离子以及少量CdOH+离子和Cd(OH)2分子存在;当pH值为9.8~13.31时,主要以Cd(OH)2分子形态存在,以及少量的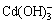 、CdOH+等离子;当pH值为11.5时,Cd(OH)2分子形态含量超过90%;当pH值为11.5~13.31时,Cd(OH)2逐渐减少,
、CdOH+等离子;当pH值为11.5时,Cd(OH)2分子形态含量超过90%;当pH值为11.5~13.31时,Cd(OH)2逐渐减少,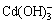 含量显著增加;当pH值大于13.31时,主要以
含量显著增加;当pH值大于13.31时,主要以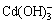 以及少量
以及少量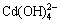 和Cd(OH)2形态存在。
和Cd(OH)2形态存在。
3 pH值对Cd(OH)2溶解度的影响
在Cd2+-H2O体系中,由于生成多种羟合配离子,将使Cd(OH)2的溶解度增加,文献[9]用条件溶度积的概念表达pH对Cd(OH)2溶解度的影响。
Cd(OH)2(s)条件溶度积KS定义为

[OH-]为游离态的羟基,[CdOH+]、[Cd(OH)2]、 、
、 同样由式(6)~(9)计算。
同样由式(6)~(9)计算。
将式(35)和(36)代入式(34),可以计算不同pH值条件下条件溶度积KS值,将计算结果用pKS对pH值作图并同时将式(5)绘于同一图中,得图3。
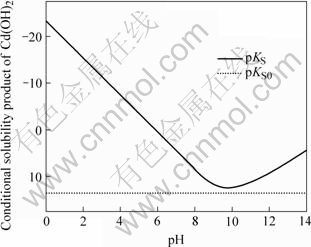
图3 Cd(OH)2条件溶度积与pH值关系
Fig.3 Relationship between conditional solubility product of Cd(OH)2 and pH value
图3中曲线表达了Cd(OH)2(s)溶解平衡时Cd(OH)2条件溶度积与pH值的关系。曲线上面所包围的面积是Cd(OH)2(s)溶解的过饱和区,会产生Cd(OH)2沉淀,其它区域为非饱和区。曲线表明,pH值在9.5~10.5范围内,Cd(OH)2的溶解度最小;当pH>10.5或pH<9.5时,Cd(OH)2的溶度积变较大,表明Cd(OH)2溶解度随pH值相应地变化而增加。同时看到,pKS0直线处在非饱和区,表明中和水解法不能将镉脱除到Cd(OH)2(s)溶度积所确定的最低镉浓度。
4 结论
1) pc—pH图描述了Cd(OH)2(s)溶解平衡时,镉的总离子平衡浓度与pH值的关系。当pH值为9.84~ 13.31时,Cd(OH)2(s)的溶解度最小。
2) αn—pH图指出了各种羟合配离子分率与pH值的关系,每种羟合配离子都有其最佳的pH值范围。
3) pKS—pH图指出了Cd(OH)2(s)的条件溶度积与pH值的关系,当pH值为9.5~10.5时,Cd(OH)2(s)的条件溶度积最小。
REFERENCES
[1] 谢红斌. 分段中和法处理重金属废水的研究[J]. 湖南有色金属, 2000, 16 (9): 91-92.
XIE Hong-bin. Study on neutralized method to treating heavy metal wastewater [J]. Hunan Nonferrous Metals, 2000, 16 (9): 91-92.
[2] 钟竹前,梅光贵,贺青蒲,张训鹏. 废水中和水解净化的理论分析[J]. 有色冶炼, 1981(3): 34-42.
ZHONG Zhu-qian, MEI Guang-gui, HE Qing-pu, ZHANG Xun-peng. Theoretical analysis of purifying wastewater by neutralized hydralyzation[J]. Theory Nonferrous Smelt, 1981(3): 34-42.
[3] 李 波. 金属碳酸盐沉淀过程的热力学分析[J]. 稀有金属与硬质合金, 2005, 32(6): 4-8.
LI Bo. The thermodynamics analysis on the deposition process of metal carbonates [J]. Rare Metals and Cemented Carbides, 2005, 32(6): 4-8.
[4] 盖庆春,郭延河,李苏琪,林华宽,王 旭,朱守荣,陈荣悌. 新型三脚架配体1,3,5-三-(n-2,5-二氮杂己烷基)-苯的合成及其Co(Ⅱ)、Ni(Ⅱ)、Cu(Ⅱ)、Zn(Ⅱ)配合物稳定性研究[J]. 南开大学学报(自然科学版), 2001, 34(2): 13-19.
GAI Qing-chun, GUO Yan-he, LI Su-qi, LIN Hua-kuan, WANG Xu, ZHU Shou-rong, CHEN Rong-ti. Thermodynamic properties of complexes of 1,3,5-tri (n-2,5-diaminonhexaane)-bebzene with Co(Ⅱ)、Ni(Ⅱ)、Cu(Ⅱ)、Zn(Ⅱ) [J]. Acta Scientiarum Naturalium Universitatis Nankaiensis, 2001, 34(2): 13-19.
[5] 高东昭,郭延河,朱守荣,林华宽,许新合. 新型酰胺金属配合物的热力学稳定性研究[J]. 高等学校化学学报, 2004, 25(4): 740-742.
GAO Dong-zhao, GUO Yan-he, ZHU Shou-rong, LIN Hua-kuan, XU Xin-he. Thermodynamic studies on a new type of carboxamide metal complexs [J]. Chemical Journal of Chinese Universities, 2004, 25(4): 740-742.
[6] Driesner T, Ha T K, Seward T M. Oxygen and hydrogen isotope fractionation by hydration complexes of Li+, Na+, K+, Mg2+, F-, Cl- and Br-: A theoretical study [J]. Geochimica et Cosmochimica Acta, 2000, 64(17): 3007-3033.
[7] Armada M P G. A program for calculation and graphic representation of conditional constants—II. Solubility products [J]. Computers Chem, 1996, 20(3): 385-387.
[8] Hou M L, Baughman G L. Predicting the precipitation of acid and direct dyes in natural waters [J]. Dyes and Pigments, 1992, 18(1): 35-46.
[9] 张祥麟,康 衡. 配位化学[M]. 长沙:中南工业大学出版社, 1986.
ZHANG Xiang-lin, KANG Heng. Coordination Chemistry [M]. Changsha: Central South University of Technology Press, 1986.
[10] Stumm W, Morgan J J. 水化学天然水体化学平衡导论[M]. 汤鸿霄, 译. 北京:科学出版社, 1987.
Stumm W, Morgan J J. Aquatic Chemistry—An Introduction Emphasizing Chemical Equilibria in Natural Waters [M]. TANG Hong-xiao, tranls. Beijing: Science Press, 1987.
[11] Trojanowicz M, Alexander P W, Hibbert D B. Flow-injection potentiometric determination of free cadmium ions with a cadmium ion-selective electrode [J]. Analytica Chimica Acta, 1998, 370: 267-278.
[12] CHEN D W, Ray A K. Removal of toxic metal ions from wastewater by semiconductor photocatalysis [J]. Chemical Engineering Science, 2002, 56: 1561-1570.
[13] 姚允斌,解 涛,高英敏. 物理化学手册[M]. 上海:上海科学技术出版社, 1985.
YAO Yun-bin, XIE Tao, GAO Ying-min. Handbook of physical chemistry [M]. Shanghai: Shanghai Science and Technology Press, 1985.
[14] 姚青松. 平衡理论在化学法处理重金属废水中的作用[J]. 污染防治技术, 1994(12): 10-12.
YAO Qing-song. The effect of the chemistry disposal of heavy metal wastewater in equilibrious theory [J]. Preventing the Pollution Technology, 1994(12): 10-12.
[15] 陈绍炎. 水化学[M]. 北京:水利电力出版社, 1989.
CHEN Shao-yan. Aquatic Chemistry [M]. Beijing: Irrigation and Electrics Press, 1989.
基金项目:国家自然科学基金资助项目(50508044);湖南省科技计划攻关重大专项资助项目(05SK1003-1)
收稿日期:2006-10-26;修订日期:2007-01-11
通讯作者:柴立元,教授,博士;电话:+86-0731-8836921;E-mail: lychai@mail.csu.edu.cn
(编辑 李艳红)