文章编号:1004-0609(2008)05-0879-05
用于基因治疗的硅纳米颗粒的制备及生物安全性评价
赵颜忠1, 2,黄艳艳1, 2,陈玉祥3,朱晒红1, 2,王国慧1, 2,周建大2,黄 东2,周科朝4
(1. 中南大学 医用材料与器械研究中心,长沙 410013;
2. 中南大学 湘雅三医院,长沙 410013;
3. 中南大学 生物科学与技术学院,长沙 410013;
4. 中南大学 粉末冶金国家重点实验室,长沙 410083)
摘 要:采用化学合成法制备用于基因治疗的易修饰的氨基化硅纳米颗粒和荧光氨基化硅纳米颗粒。用透射电镜和Zeta电位仪对纳米颗粒进行分析,并参照ISO7406技术报告中的相关标准,对纳米颗粒的生物相容性进行一系列体内及体外实验,包括细胞毒性实验、动物急性毒性实验、动物生殖毒性实验。结果表明:新研制的氨基化硅纳米颗粒的粒径为40 nm左右;在中性条件下,氨基化硅纳米颗粒的表面净正电荷约为16 mV;纳米颗粒对细胞生长无明显影响,对实验动物无生长和生殖毒性,与对照组相比无显著性差异。
关键词:氧化硅;纳米颗粒;基因治疗;非病毒载体;生物安全性
中图分类号:TB 39 文献标识码:A
Preparation and biological savety evaluation of
silicon nanoparticles for gene therapy
ZHAO Yan-zhong1, 2, HUANG Yan-yan2, CHEN Yu-xiang3, ZHU Shai-hong1, 2, WANG Guo-hui1, 2,
ZHOU Jian-da2, HUANG Dong2, ZHOU Ke-chao4
(1. Research Center for Medical Material and Instruments, Central South University, Changsha 410013, China;
2. The Third Xiangya Hospital, Central South University, Changsha 410013, China;
3. School of Biological Science and Technology, Central South University, Changsha 410013, China;
4. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China)
Abstract: Silica nanoparticles and fluorescence silica nanoparticles with amino group modified on their surface were prepared as gene carrier. The size and Zeta potential of nanoparticles were tested by electron microscopy and Zeta potential measurement. According to the relate methods and regulations of medical devices prescribed by the International Organization for Standardization(ISO), to evaluate their biological compatibility, their cytotoxicity, general acute toxicity and reproductive toxicity assessment were performed. The results show that the newly developed silica nanoparticles are 40 nm in size by electron microscopy, and Zeta potential of its surface is 16 mV on the condition of neuter of pH value. Toxicity testing in vitro and in vivo reveals that it has no significant influence for silica nanoparticles when they are cultured with normal cells and are injected into mice by tail-vein intravenous administration to observe if they affect experimental animal reproductivity.
Key words: silica; nanoparticles; gene therapy; non-viral carrier; biological safety
随着纳米生物技术的迅速发展,纳米颗粒本身独特的性质(如表面效应、小尺寸效应、宏观量子隧道效应等)使得纳米颗粒用于非病毒型的基因转移载体已成为目前的研究热点,如树枝状高聚物(Dendrimers)[1]、磷酸钙纳米颗粒(Calcium phosphate nanoparticles)[2]、硅纳米颗粒(Silica nanoparticles)[3]、羟基磷灰石纳米颗粒(Hydroxyapatite nanoparticles)[4]、四氧化三铁纳米颗粒(Magnetic nanoparticle)[5-6]等,表现出低毒、大容量、易制作等优越性,尤其是其具有明确的化学结构,使设计和研制新的更理想的载体系统成为可能[7-9],但纳米颗粒仍与其他非病毒型载体一样具有低转染效率,无靶向性等缺陷限制了其在临床上的应用。本文作者在前期研究单纯硅纳米颗粒作为基因转移载体的基础上[10],利用化学合成的方法制备易于修饰的表面富含氨基的硅纳米颗粒,并对其生物安全性进行研究,以便为下一步构建一种能高效、安全、靶向、简便的新型硅纳米基因转导系统打下基础。
1 实验
1.1 实验材料
材料为:正硅酸乙酯(TEOS)、N-(β-氨乙基)-γ-氨丙基三乙氧基硅烷、钌吡啶配合物、琼脂糖,购自Sigma公司;环已烷、正己醇、丙酮、氨水(氨水含量≥28%),均为国产,分析纯;纯小牛血清、胎牛血清,为杭州四季青公司产品;其他试剂,均为市购;细胞株HT1080 和Hela,购自中国典型培养物保藏中心(中国武汉)。自制纯水,符合中国药典2000年版注射用水项下的要求;昆明种小白鼠,由中南大学实验动物学部提供,湖南省医学实验动物管理委员会颁发的医学实验动物合格证书(小白鼠昆明种)(医动字第024号)。
1.2 表面氨基化的硅纳米颗粒制备
采用油包水的微乳液方法将环己烷、表面活性剂TritonX-100和正己醇按体积比4.2?1?1混合均匀, 以适量的水作为分散相,磁力搅拌1 h,然后以一定比例加入正硅酸乙酯(TEOS)、N-(β-氨乙基)-γ-氨丙基三乙氧基硅烷(氨基化试剂)和适量氨水(催化剂),在磁力搅拌下于室温反应24 h,反应产物在10 000 r/min下离心分离10 min,分别用丙酮、75%酒精和去离子双蒸水洗脱,超声分散,高压蒸汽灭菌。此外,在不改变以上各成分比例的情况下,加入钌吡啶配合物[Ru(Ⅱ)(bpy)3]2+水溶液,制备包裹有[Ru(Ⅱ)(bpy)3]2+荧光氨基化的硅纳米颗粒。对制备的氨基化的硅纳米颗粒混悬液进行颗粒形态、大小、均匀度进行考察,对其表面进行Zeta电位检测,并按中国生物制品规程(2000年版)进行无菌试验和内毒素检查,全面考察制备工艺。
1.3 氨基化的硅纳米颗粒的细胞毒性测定
将1×105/mL Hela细胞接种于96孔组织培养板中,每孔100 μL,置于温度为37 ℃、浓度为5% CO2的培养箱预培养,使细胞汇合度达60%~70%,然后,加入50 μL不同浓度的氨基化硅纳米颗粒混悬液,震荡混均,继续培养24,48和96 h后进行MTT测定。将MTT溶液过滤除菌,以正常生长细胞为对照组,每孔加入5 g/L MTT(保存液)20 μL,培养4 h,弃上清液,每孔加入乙醇和二甲基亚砜(DMSO)混合液(1?1) 100 μL,振荡5 min,每孔取100 μL置于OD测定板,测不同时间的OD值,波长为595 nm。采用比色法确定有活力的细胞数,采用SPSS 10.0统计软件进行方差分析。
1.4 氨基化的硅纳米颗粒的急性毒性实验
取健康无病昆明种小白鼠120只,雌雄各半,每只鼠质量为17~20 g,当天下午禁食16 h(不禁水),随机分成对照组和实验组,每组20只;经内毒素检测合格的硅纳米颗粒1次给予每只鼠0.4 mL(最大体积),按照一定的浓度梯度的硅纳米颗粒悬液尾静脉注射小白鼠,硅纳米颗粒悬液的注射剂量按正常剂量的等比数例注射,观察2周内动物的死亡情况及毒性反应[11]。
1.5 硅纳米颗粒的动物生殖毒性实验
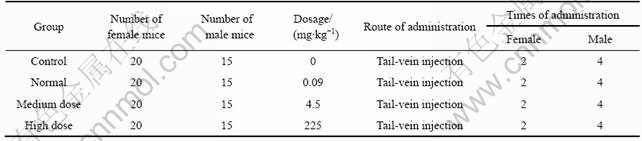
根据硅纳米颗粒的动物急性毒性实验实验结果,拟定低、中、高剂量(分别为90 μg /(kg?d),4 500 μg/(kg?d)和225 mg/(kg?d)组和生理盐水对照组,每组35只昆明种小白鼠(雄15、雌20只)。将雄、雌小鼠各随机分为3个给药组和1个对照组,每组含15只雄鼠和20只雌鼠,每天通过尾静脉注射已配制好的硅纳米颗粒悬液(见表1),分别连续给药9周和12周,每周给药2次,然后,将雄鼠和雌鼠以1?1合笼,共合笼5 d,合笼期间连续给药,雄鼠给药至11周,雌鼠则继续给药至怀孕的第15 d。根据新药临床前安全性评价标准判断其生殖毒性[12-13]。雌雄鼠体重,黄体数,着床数,活胎数,胎仔体重等平均数均用F检验。交配率和受孕率用χ2检验。
表1 硅纳米颗粒悬液的小白鼠给药方案
Table 1 Administration program of silica nanoparticles suspension in little white mice
2 实验结果
2.1 氨基化硅纳米颗粒的电镜及其Zeta电位仪检测
对所制备的氨基化的硅纳米颗粒进行优化超声处理后电镜检测结果如图1所示。可见,硅纳米颗粒形态规则,粒径为40 nm左右,较前期制备的硅纳米颗粒的粒径更小。采用Zeta电位仪对其进行Zeta电位检测,在检测之前用精密pH试纸测得氨基化的硅纳米颗粒悬液的pH值呈中性,该纳米颗粒的Zeta电位为净正电,达16 mV左右,而单纯的硅纳米颗粒在pH值呈中性时为净负电。
2.2 批量制备的硅纳米颗粒内毒素检测结果
将实验样品反应管及阳性对照组从水浴箱中缓慢取出,并倒转180?,阳性管管内呈坚实凝胶样,而实验样品管未呈凝胶样,认为内毒素符合药典标准。

图1 粒径为20~50 nm的硅纳米颗粒的SEM像
Fig.1 SEM image of silica nanoparticles with diameters of 20-50 nm
2.3 氨基化的硅纳米颗粒的细胞毒性测定
以MTT显色法检测96孔板Hela细胞在不同时间的吸光度(OD),根据存活细胞数量与吸光值呈正比的原理,按公式计算得知高、中、低剂量组和非处理对照组的细胞存活率分别为80%,85%,78%和82%。说明各剂量组对细胞的正常生长无明显影响。
2.4 氨基化的硅纳米颗粒的急性毒性实验结果
将实验组分成5组,在保证每只小白鼠注射0.4 mL液体的情况下,按不同的浓度将纳米颗粒稀释。第一组经腹腔注射的纳米颗粒剂量为225.0 mg/kg,第二组经尾静脉注射的纳米颗粒剂量为112.5 mg/kg,第三组经腹腔注射的纳米颗粒剂量为56.3 mg/kg,第四组经腹腔注射的纳米颗粒剂量为25.0 mg/kg,第五组经腹腔注射的纳米颗粒剂量为5.0 mg/kg,对照组经尾静脉注射的生理盐水0.4 mL/只,在饲养2个星期后,实验组及对照组小白鼠均未出现明显的毒性反应及死亡情况(见表2)。
2.5 氨基化的硅纳米颗粒的生殖毒性实验结果
2.5.1 纳米颗粒对小白鼠的影响
各剂量组在交配给药前,交配给药期间未见有死亡发生,解剖时也未见有明显的异常。各剂量组雄鼠的交配率及交配后致雌鼠怀孕率与对照组相比均无显著差异(见表3)。
脏器质量检查,给药组睾丸和附睾平均质量与对照组相比有差异,但脏器系数无显著性的差异(P>0.05),且3个实验剂量组之间不具剂量反应关系(见表4)。
2.5.2 硅纳米颗粒对胎仔的影响
对剂量为90 μg/(kg?d),4 500 μg/(kg?d),225 mg/(kg?d)的组各随机抽查了1窝胎仔的外形,未见胎仔形态异常。胎仔内脏软组织,未见明显的异常,其窝数和胎仔数的发生率与对照组相比均无差异(P>0.05)。对胎仔进行骨骼检查未发现胎仔胸骨节第一中心及耻骨未骨化的现象。各剂量组均无明显软骨畸形。
3 讨论
如何选择合适的载体,使目的基因稳定、有效地表达,是基因治疗成功的关键因素之一。鉴于病毒载体的诸多缺陷,非病毒载体已成为基因治疗研究中的热点课题,也是基因治疗今后临床应用的发展方向之一。随着近几年对纳米生物技术的开展和深入,人们开始认识到一些纳米材料的生物学特性,如表面效应、小尺寸效应、宏观量子隧道效应和量子尺寸效应等。因此,利用纳米颗粒的特殊性质可方便地在其表面结合生物大分子,成为最有应用前景的非病毒基因高效载体。
根据前期研究[10]采用油包水形成微胶囊与正硅酸乙酯(TEOS)水解的方法制备了一批粒径为50 nm左右的单纯的硅纳米颗粒,但该单纯的硅纳米颗粒本身并不带有净正电荷,尤其在中性条件下。因此,在此基础上,根据文献[14-15]的报道,进一步研究和分析其制备原理和工艺过程及其可能作为DNA转移载体的条件,采用正硅酸乙酯(TEOS)和N-(β-氨乙基)-γ-氨丙基三乙氧基硅烷(氨基化试剂)在微乳液体系环境中同步水解法直接制得表面富有氨基的硅纳米颗粒,产品外观为白色的悬浮液或白色粉末。利用该方法制备纳米颗粒的实验装置要求简单,操作容易,不需要高温高压等其他苛刻的实验条件,并且摸索出水与表面活性剂的摩尔比和正硅酸乙酯与氨水的摩尔比是控制纳米颗粒粒度的关键条件。此外,氨水的浓度和正己醇也是一个重要条件。在不改变以上各成分比例的情况下,加入钌吡啶配合物[Ru(Ⅱ)(bpy)3]2+水溶液,制备包裹有[Ru(Ⅱ)(bpy)3]2+荧光氨基化的硅纳米颗粒,在冷冻抽干后呈淡红色粉末,在荧光显微镜下颗粒呈红色,并且用紫外线照射1 h,颗粒的荧光强度没有明显衰退(未附图)。同时,采用Zeta电位仪对其进行Zeta电位检测,在检测之前用精密pH试纸测得氨基化的硅纳米颗粒悬液的pH值呈中性,该纳米颗粒的Zeta电位为净正电,达16 mV左右,而单纯的硅纳米颗粒在pH呈中性时为净负电。因此,为所制备的氨基化硅纳米颗粒用于基因转导载体提供了理论依据。
表2 硅纳米颗粒悬液小白鼠急性毒性试验结果
Table 2 Acute toxicity test results in little white mice after silica nanoparticles suspension injection
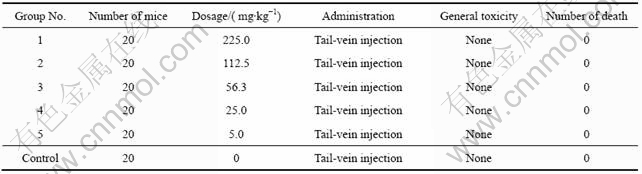
表3 小鼠雌雄合笼后的雌鼠受孕率结果(N = 20)
Table 3 Results of fecundation rate of female little mice (N = 20)

表4 雄性小鼠睾丸及副睾质量的比较
Table 4 Comparison of testicle and epididymis mass between tests and control groups

在本研究体系中,本文作者选用的无机物硅纳米颗粒作为基因转导载体,具有以下特点和优势:1) 纳米颗粒制备简便、成本低,无需特殊的条件和设备;2) 纳米颗粒在制备过程中通过改变有关实验反应参数,可很容易控制纳米颗粒的粒径,粒径分布也较均匀;3) 制备的纳米颗粒粒径最小可达20 nm左右,可很容易渗出血管,被细胞吞噬;4) 在中性条件下,纳米颗粒表面净正电荷电压为约16 mV,可通过静电作用有效地与DNA结合形成复合体;5) 纳米颗粒表面富含氨基,能够有效地进行表面修饰,连接具有生物活性的物质,提高其介导基因转移的靶向性;6) 纳米颗粒具有良好的生物相容性,无细胞毒性和实验动物生长生殖毒性,具有临床应用前景。如何提高氨基化硅纳米颗粒基因转染效率和靶向性将是下一步的研究目标。
REFERENCES
[1] TANG M X, REDEMANN C T, SZOKA F. In vitro gene delivery by degraded polyamidoamine dendrimers[J]. Bioconjug Chem, 1996, 7(6): 703-714.
[2] ROY I, MITRA S, MAITRA A, MOZUMDAR S. Calcium phosphate nanoparticles as novel non-viral vectors for targeted gene delivery[J]. Int J Pharm, 2003, 250(1): 25-33.
[3] O’FARRELL N, HOULTON A, HORROCKS B R. Silicon nanoparticles: applications in cell biology and medicine[J]. Int J Nanomedicine, 2006, 1(4): 451-472.
[4] ZHU S H, HUANG B Y, ZHOU K C, HUANG S P, LIU F, LI Y M, XUE Z G, LONG Z G. Hydroxyapatite nanoparticles as a novel of gene carrier[J]. Journal of Nanoparticle Research, 2004, 6: 311-317.
[5] ALEXIOU C, JURGONS R, SELIGER C, IRO H. Medical applications of magnetic nanoparticles[J]. J Nanosci Nanotechnol, 2006, 6(9/10): 2762-2768.
[6] XIANG J J, TANG J Q, ZHU S G, LU H B, SHEN S R, LI X L, TANG K, ZHOU M, LI G Y. IONP-PLL: a novel non-viral vector for efficient gene delivery[J]. J Gene Med, 2003, 5(9): 803-817.
[7] LI S, HUANG L. Noviral gene therapy: promises and challenges[J]. Gene Ther, 2000, 7(1): 31-34.
[8] WAGNER E. Application of membrane active peptides for nonviral gene delivery[J]. Adv Drug Deliv Rev, 1999, 38(3): 279-289.
[9] HARUSH-FRENKEL O, DEBOTTON N, BENITA S, ALTSCHULER Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway[J]. Biochem Biophys Res Commun, 2007, 353(1): 26-32.
[10] CHEN Y, XUE Z, ZHENG D, XIA K, ZHAO Y, LIU T, LONG Z, XIA J. Sodium chloride modified silica nanoparticles as a non-viral vector with a high efficiency of DNA transfer into cells[J]. Curr Gene Ther, 2003, 3(3): 273-279.
[11] 徐叔云. 药理学实验[M]. 北京: 人民卫生出版社, 1982: 902.
XU Shu-yun. Pharmacology experiment[M].Beijing: People’s Medical Press, 1982: 902.
[12] 袁伯俊, 王治乔. 新药临床前安全性评价与实践[M]. 北京: 军事医学科学出版社, 1997.
YUAN Bo-jun, WANG Zhi-qiao. Preclinical safety evaluation and practice of pharmaceuticals[M]. Beijing: Military Medical Sciences Press, 1997.
[13] 张天宝. 发育毒理学研究方法和实验技术[M]. 北京: 北京医科大学出版社, 2000.
ZHANG Tian-bao. The method of study and the technology of experiment of developmental technology[M]. Beijing: Beijing Medical University Press, 2000.
[14] ARRIAGADA F J, OSSEO-ASARE K. Synthesis of nanosize silica in a nonionic water-in-oil microemulsion: Effects of the water/surfactant molar ratio and ammonia concentration[J]. J Colloid Interface Sci, 1999, 211(2): 210-220.
[15] OSSEO-ASARE K, ARRIAGADA F J. Growth kinetics of nanosize silica in a nonionic water-in-oil microemulsion: A reverse micellar pseudophase reaction model[J]. J Colloid Interface Sci, 1999, 218(1): 68-76.
基金项目:湖南省自然科学基金资助项目(06JJ50072);湖南省科学计划资助项目(05FJ3015)
收稿日期:2007-09-20;修订日期:2008-01-16
通讯作者:周科朝,教授,博士;电话:0731-8830464;E-mail: zhoukechao@mail.csu.edu.cn
(编辑 龙怀中)