
High temperature oxidation behavior of electroconductive TiN/O′-Sialon ceramics prepared from high titania slag-based mixture
JIANG Tao1, 2, XUE Xiang-xin1, 2, LI Zhe-fu1, 2, DUAN Pei-ning1, 2
1. School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China;
2. Institute of Metallurgical Resources and Environmental Engineering, Northeastern University,Shenyang 110819, China
Received 2 March 2011; accepted 25 May 2011
Abstract: The oxidation behavior of electroconductive TiN/O′-Sialon ceramics prepared using high titania slag as main starting material was studied at 1 200-1 300 ℃ in air. The isothermal and non-isothermal oxidation processes were investigated by DTA-TG. Phase compositions and morphologies of the oxidized products were analyzed by XRD, SEM and EDS. The results indicate that the oxidation of TiN and O′-Sialon occurs at about 500 ℃ and 1 050 ℃, respectively. After oxidation at 1 200-1 300 ℃, a protective scale that consists of Fe2MgTi3O10, SiO2 and TiO2 is formed on the surface of the materials, which effectively prevents the oxidation process. The formation of a protective scale is relative to TiN content and apparent porosity of the samples, the amount of SiO2 and amorphous phase in the oxidation product. At the initial oxidation stage, the oxidation kinetics of the materials follows perfectly the linear law with the apparent activation energy of 1.574×105 J/mol, and at the late-mid stage, the oxidation of the samples obeys the parabolic law with the apparent activation energy of 2.693×105 J/mol. With the increase of TiN content, mass gain of the materials increases significantly.
Key words: TiN/O′-Sialon; high titania slag; electroconductive ceramics; oxidation behavior
1 Introduction
O′-Sialon (Si2-xAlxO1+xN2-x, 02/O′-Sialon [5], TiN/β′-O′-Sialon [6], TiN/O′-Sialon [7], etc, multiphase ceramics are prepared by different methods to improve the strength and toughness of O′-Sialon through granule dispersion strengthening and phase transformation toughening. All of these materials show better mechanical, physical and chemical properties than single-phase O′-Sialon. However, these composites are hard, brittle and cannot be machined efficiently by conventional diamond tools. As a result, electric-discharge machining (EDM) would be an attractive alternative technique. Although EDM has higher efficiency, higher machining accuracy than traditional machining and can process complex shape of parts, decrease material wastage and processing cost, it requires materials with electrical conductivity higher than 100 Ω·cm, which is usually not obtainable for most ceramics. To match this requirement, considerable work has been conducted to improve the electrical conductivity of ceramics. One of efforts is through the composite approach, i.e., by adding a conductive second phase into ceramics. As a reinforcing material, TiN offers several unique advantages such as high melting temperature (2 950 ℃), excellent stiffness (600 GPa), high hardness, good chemical durability, high electrical conductivity (4.6×106 Ω-1·m-1) [8], and the chemistry compatibility with O′-Sialon ceramics. As a result, the addition of TiN to O′-Sialon ceramics is expected to increase not only the toughness but also their electrical conductivity. Electroconductive TiN/O′-Sialon ceramics which are modified by TiN can be processed by EDM and are expected to be used as electrode or heating materials.
Although O′-Sialon has excellent oxidation resistance, TiN as a nitride is readily oxidized at high temperature. So, it is important to investigate the high temperature oxidation behavior of TiN/O′-Sialon for achieving their application. Recently, the oxidation behaviors of O′-Sialon [9], β′-Sialon [10], Si3N4 [11] and TiN/Si3N4 [12] were studied. But there were no reports about TiN/O′-Sialon ceramic. Therefore, the oxidation behavior of TiN/O′-Sialon ceramics prepared using high titania slag as the staring material in air at high temperature and the effect of TiN content on the oxidation process are studied in this work.
2 Experimental
Electroconductive TiN/O′-Sialon ceramics were prepared by two-step method in this work. First, TiN/O′-Sialon powder was synthesized using high titania slag, silica fume and bauxite chamote as the starting materials by carbothermal reduction–nitridation in a nitrogen atmosphere with a N2 flow rate of 400 mL/min at 1400 ℃ for 7 h. The phases of the products are composed of O′-Sialon, TiN, small amounts of β′-Sialon and the unreacted TiO2. Subsequently, the powder was pressed into pellets with 15 mm in diameter at 200 MPa, then put into the graphite crucible and buried with powder (the mole ratio of Si3N4 to SiO2 is 1:1). TiN/O′-Sialon ceramics were prepared by pressureless sintering at 1500 ℃ for 2 h. The samples were marked with T10, T20, T30, T40 and the number indicated the content of TiO2 in the starting materials. Phase compositions and properties of TiN/O′-Sialon ceramics are shown in Table 1.
Setsys Evolution16 comprehensive thermal analyzer was employed to investigate the starting temperature and reaction course of the oxidation of the samples in air. The heating rate was 10 ℃/min to confirm the oxidation temperature. Specimens with regular shape were cut from the sintered pellets, wet polished, ultrasonically cleaned, dried, and then measured the surface area. Oxidation experiments were conducted using a MoSi2 resistance furnace at different temperatures in air. The mass gain was measured by MettlerA04 type electronic balance whose precision was 0.1 mg. In the whole oxidation process, the air with a flow rate of 80 mL/min was continuously blown into the furnace. After oxidation at 1200, 1260 and 1300 ℃ for 2 h, the samples were removed and cooled to the room temperature.
Phase identification of the oxidized layers was performed by D/MAX-RB X-ray diffractometer (XRD) using nickel-filtered Cu Ka radiation, and the morphologies of surface and cross section were observed by SSX-550 scanning electron microscope (SEM). The element analysis of characteristic grain on the oxidized surface was carried out by energy dispersion spectroscopy (EDS).
3 Results and discussion
3.1 Oxidation process analysis
The oxidation processes of TiN/O′-Sialon ceramics are composed of TiN and O′-Sialon. The oxidation reactions are as follows:
TiN(s)+O2(g)=TiO2(s)+0.5N2(g) (1)
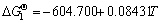 , 298-1943 K [13]
, 298-1943 K [13]
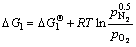
Si1.7Al0.3O1.3N1.7(s)+1.275O2(g)=
1.7SiO2(s)+0.15Al2O3(s)+0.85N2(g) (2)
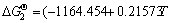 , 298-1685 K [14]
, 298-1685 K [14]
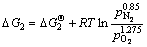
Thermodynamic analysis results show that the variation in the Gibbs free energy of reactions (1) and (2) is far less than 0 at room temperature up to 1673 K in air, which implies that the oxidation of both TiN and O′-Sialon is thermodynamically favorable. Therefore, thermal-analysis tests are necessary to determine the exact starting temperatures of the two reactions.
Figure 1 shows the DTA-TG curves of TiN/O′- Sialon in air. There are four obvious exothermic peaks in DTA curve for TiN/O′-Sialon, in the meanwhile the obvious mass change in TG curve. The beginning temperature of the first exothermic peak is 500 ℃ and the forth one is 1050 ℃. According to Ref. [15], the first peak is exothermic peak of TiN and the forth one is O′-Sialon. The other two peaks could come from the oxidation of impurity phases. According to the analysis results of DTA-TG, the isothermal oxidation experiments were carried out at 1200, 1260 and 1300 ℃ for 2 h.
Table 1 Phase compositions and properties of TiN/O′-Sialon prepared at 1 500 ℃ for 2 h

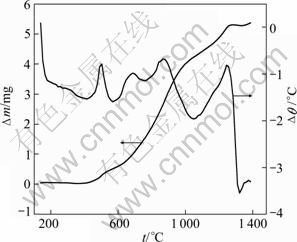
Fig. 1 DTA-TG curves for oxidation of TiN/O′-Sialon in air
3.2 Oxidation behavior at high temperature
Figure 2 shows the XRD pattern of the oxidized surface of the sample T30 at 1260 ℃ for 2 h. The oxidized layer mainly consists of Fe2MgTi3O10, TiO2 and SiO2. The morphologies of the surface and the cross-section of the sample T30 which is oxidized at 1200 and 1260 ℃ for 2 h are shown in Fig. 3. Large amounts of lath-shaped Fe2MgTi3O10 grains are observed and confirmed by XRD and EDS analysis (Fig. 4). The Fe elements mainly derived from impurity phases of the starting materials. As shown in Figs. 3(a) and (c), these lath-shaped grains are covered by a layer of SiO2 and glass phases and no obvious pores can be observed. With the temperature increasing, the lath-shaped grains grow continually. Three obvious layers can be observed on the cross-section of the sample T30 after oxidation at 1200 and 1260 ℃ (Figs. 3(b) and (d)). The outer layer is a protective scale layer formed by molten oxide, and its thickness is about 90 μm. The protective scale formed at 1200 ℃ is more compact than that formed at 1260 ℃. The middle layer is solid-phase oxide layer and part of which has been oxidized before the outer protective scale formed. Many pores caused by the diffusion of metal ions to the outside layer were observed. The inner layer is unoxidized TiN/O′-Sialon matrix.
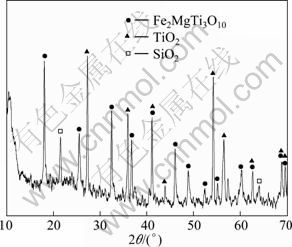
Fig. 2 XRD patterns of oxidized sample T30 at 1 260 ℃ for 2 h in air
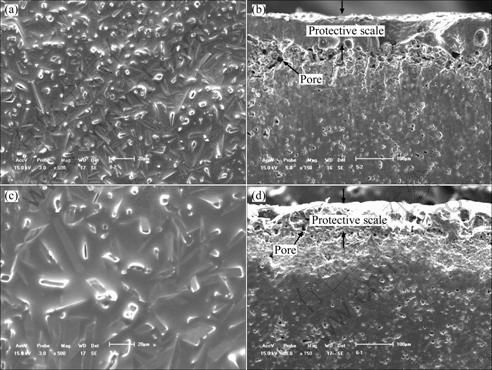
Fig. 3 SEM images of surface and cross-section of sample T30 after oxidation for 2 h in air: (a) 1200 ℃, surface; (b) 1200 ℃, cross-section; (c) 1260 ℃, surface; (d) 1260 ℃, cross-section
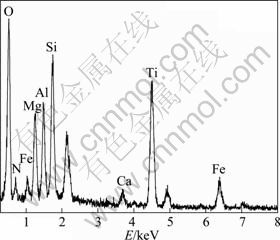
Fig. 4 EDS spectrum of lath-shaped grain in oxidation layer of sample T30
Figure 5 shows the isothermal TG curves of the sample T30 at 1200, 1260 and 1300 ℃. As shown in Fig. 5, the mass gain increases obviously at the initial oxidation stage, which indicates that on the sample surface, an effective protective scale cannot form. At this stage, the chemical reaction is the rate-limiting step of the oxidation process and the oxidation of the samples follows the linear law with the apparent activation energy of 1.574×105 J/mol (Fig. 6(a)). With the oxidation time prolonging, the thickness of protective scale increases and closes the surface pores and prevents the oxygen from diffusing to the interior of the sample through the oxidation layer. In that case, the oxidation kinetics of the materials can be described by a class parabolic law with an apparent activation energy of 2.693×105 J/mol (Fig. 6(b)), which means that the diffusion of O2 through the oxidized layer to the reaction layer or the diffusion of metal ions through the product layer to the outer layer is the rate-limiting step of the oxidation process. The researches have shown that the oxidation kinetics of Si3N4 and Si2N2O matrix ceramics at high temperatures also followed the parabolic rule [16-18].
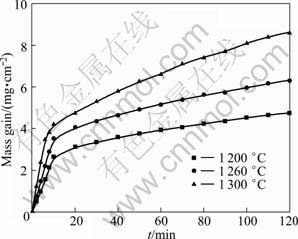
Fig. 5 Isothermal TG curves for sample T30 at different temperatures
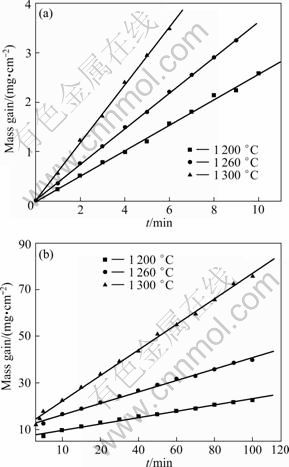
Fig. 6 Oxidation kinetic curves of samples: (a) Initial stage; (b) Late-mid stage
3.3 Effect of TiN content on oxidation process
Figure 7 shows the XRD patterns of the oxidized surfaces of the samples T10-T40 at 1260 ℃ for 2 h. As indicated in Fig. 7, Fe2MgTi3O10, TiO2 and SiO2 are found in all the samples, but their contents are different. The SiO2 content of the oxidation layer of sample T10 is high and contains large amounts of amorphous phase. The SiO2 content in sample T20 obviously decreases, but the product still contains large amounts of amorphous phases. The SiO2 content of sample T30 and amorphous phase are little, but the TiO2 content obviously increases. Fe2MgTi3O10 is only phase in sample T40, and SiO2 and amorphous phase do not exist.
Figure 8 shows the isothermal TG curves of the samples T10-T40 at 1 260 ℃. As shown in Fig. 8, with the increase of TiN content, the mass gain per unit area increases obviously in the initial stage of the oxidation process, which is due to the fact that TiN is easier to be oxidized at lower temperature. Although the porosity in samples T10 is high (see Table 1), large amounts of molten SiO2 formed at the initial oxidation stage close the pores on the surface rapidly and prevent the oxygen from diffusing into the interior of the sample. So, the passive oxidation occurs and the mass gain is little in the initial oxidation stage. The molten SiO2 is reserved as the amorphous phase in the following cooling processes. Although the molten SiO2 of sample T20 on the surface decreases obviously in the initial oxidation stage, the pores on the sample surface still are closed rapidly due to lower porosity compared with sample T10. TiN content of sample T20 is higher than that of sample T10, so the mass gain of the oxidation process is higher. Although the content of SiO2 generated on the sample surface is little, its porosity is very low and the large amounts of TiO2 generated on the sample surface reduce the liquid phase formation temperature, which effectively leads to formation of the protective scale. The mass gain in the initial oxidation stage is obvious caused by the high TiN content in the sample T30. There is an effective protective scale formed on the surface of sample T40 due to high porosity of the material and no molten SiO2 and TiO2 produced, so the oxidation process aggravated. As a whole, the amount of SiO2 and TiO2 formed by O′-Sialon and TiN oxidation and apparent porosity of the materials have a great influence on the formation of a protective scale, but the effect of Fe2MgTi3O10 is not significant.
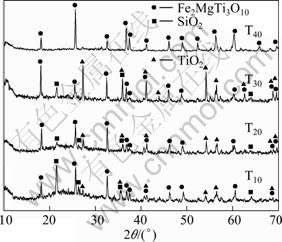
Fig. 7 XRD patterns of samples T10-T40 after oxidation at 1260 ℃ for 2 h
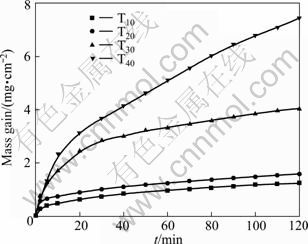
Fig. 8 Isothermal TG curves of samples T10-T40 at 1 260 ℃
4 Conclusions
1) The oxidation processes of TiN/O′-Sialon ceramics are composed of TiN and O′-Sialon oxidations which begin at about 500 ℃ and 1050 ℃, respectively.
2) At 1200-1300 ℃, the oxidation kinetics of the materials at the initial stage follows a linear law and no effective protective scale is formed and the apparent activation energy is 1.574×105 J/mol. The oxidation kinetics at the late-mid stage obeys a parabolic law and an effective protective scale is generated on the specimen surface. In that case, the diffusion of the oxygen through gradually closed pores is considered to be the rate- limiting step and the apparent activation energy of the oxidation is 2.693×105 J/mol, exhibiting good oxidation resistance.
3) The formation of a protective scale is connected with the amounts of SiO2 and amorphous phases generated on the oxidized surface and apparent porosity of the materials. With the increase of TiN content, mass gain of the materials increases significantly.
References
[1] ZENG Han-min. Review of high-tech materials [M]. Beijing: Science and Technology Press, 1993. (in Chinese)
[2] YANG J, XUE X X, JIANG T, XIE P, WANG M. Preparation of in-situ TiO2/O′-Sialon multiphase ceramics by selective-oxidation[J]. International Ceramics, 2006, 32(5): 533-538.
[3] ASADA S, UEKI M, SUGIYAMA M. O'-β'-Sialon ceramics [J]. Journal of Materials Science, 1993, 28: 3789-3792.
[4] BOSKOVIC S, TIEN T Y. Formation of O'/β'-Sialon in the presence of yttria [J]. Key Engineering Materials, 1994, 89-91: 381-386.
[5] WANG Xi-dong, BAO Hong, LI Wen-chao. Oxidation of O'-SiAlON-ZrO2 composite ceramics [J]. Journal of University of Science and Technology Beijing, 2001, 8(1): 43-47. (in Chinese)
[6] DUAN R G, ROEBBEN G, VLEUGELS J, van der BIEST O. Optimization of microstructure and properties of in situ formed β-O-Sialon–TiN composite [J].Materials Science and Engineering A, 2006, 427(1): 195-202.
[7] JIANG Tao, XUE Xiang-xin, DUAN Pei-ning, DU Gang. Fabrication and properties of TiN/O?-Sialon electroconductive composites [J].Acta Metallurgica Sinica, 2008, 43(2): 131-136. (in Chinese)
[8] MO Wei, DENG Guo-zhu. Titanium metallurgy [M]. Beijing: Metallurgical Industry Press, 1998. (in Chinese)
[9] HOU X M, CHOU K C, LI F S. Some new perspectives on oxidation kinetics of Sialon materials [J]. Journal of the European Ceramic Society, 2008, 28: 1243-1249.
[10] HOU X M, YUE C S, SINGH A K, ZHANG M, CHOU K C. Morphological development and oxidation of elongated β-Sialon material [J]. Corrosion Science, 2011, 53: 2051-2057.
[11] HOU Xin-mei, CHOU Kuo-Chih, HU Xiao-jun, ZHAO Hai-lei. A new measurement and treatment for kinetics of isothermal oxidation of Si3N4 [J]. Journal of Alloys and Compounds, 2008, 459: 123-129.
[12] ZOU Bin, HUANG Chuan-zhen, CHEN Ming, GU Mei-lin, LIU Han-lian. High-temperature oxidation behavior and mechanism of Si3N4/Si3N4w/TiN nanocomposites ceramic cutting tool materials [J]. Materials Science and Engineering A, 2007, 459: 86-93.
[13] LIANG Ying-jiao, CHE Yin-chang. Thermodynamic data notebook of inorganic [M]. Shenyang: Northeastern University Press, 1993. (in Chinese)
[14] ZHANG Hai-jun, LI Wen-chao, ZHONG Xiang-chong. Pressureless sintering and thermodynamic analysis of O'-Sialon-ZrO2-SiC composites [J]. Journal of Chinese Ceramic Society, 1999, 27(1): 41-47. (in Chinese)
[15] XIE Peng. Study on preparation process, structure and properties of in-situ synthesized TiN/O'-Sialon composite [D]. Shenyang: Northeastern University, 2000. (in Chinese)
[16] HOUJOU K, ANDO K, CHU M C, LIU S P, SATO S. Effect of sintering additives on the oxidation behavior of Si3N4 ceramics at 1 300 ℃ [J]. Journal of the European Ceramic Society, 2005, 25: 559-567.
[17] MAZEROLLES L, FELDHOFF A, TRICHET M F, MONIKA B R. Oxidation behaviour of Si3N4–TiN ceramics under dry and humid air at high temperature [J].Journal of the European Ceramic Society, 2005, 25(10): 1743-1748.
[18] MACKENZIE K J D, SHEPPARD C M, BARRIS G C, MILLS A M, SHIMADA S, KIYONO H. Kinetics and mechanism of thermal oxidation of Sialon ceramic powders [J]. Thermochemica Atca, 1998, 318: 91-100.
TiN/O′-Sialon导电陶瓷的高温氧化行为
姜 涛1, 2, 薛向欣1, 2, 李哲夫1, 2, 段培宁1, 2
1. 东北大学 材料与冶金学院,沈阳 110819;
2. 东北大学 冶金资源与环境工程研究所,沈阳 110819
摘 要:以高钛渣为主要原料制备的TiN/O′-Sialon导电陶瓷为研究对象,研究TiN/O′-Sialon材料在1200~1300 ℃下的抗空气氧化行为。采用DTA-TG法研究材料的恒温和变温氧化过程,利用X射线衍射、扫描电镜和能谱分析检测方法对氧化产物的相组成和显微结构进行表征。结果表明:TiN和O′-Sialon的氧化分别从500 ℃和1 050 ℃左右开始进行。在1200~1300 ℃下氧化后的材料表面可形成由Fe2MgTi3O10、SiO2和TiO2组成的较致密的保护膜,其形成主要与材料中TiN含量、氧化产物中SiO2和非晶相数量以及材料显气孔率有关。材料氧化前期遵循直线规律,中后期遵循抛物线规律,其表观活化能分别为1.574×105 J/mol和2.693×105 J/mol。增加材料中TiN的含量从而导致材料单位面积氧化质量显著增加。
关键词:TiN/O′-Sialon;高钛渣;导电陶瓷;氧化行为
(Edited by LI Xiang-qun)
Foundation item: Project (2007CB613504) supported by the National Basic Research Program of China; Project (20070145041) supported by the Specialized Research Fund for the Doctoral Program of Higher Education, China
Corresponding author: JIANG Tao; Tel: +86-24-83687719; E-mail: jiangt@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(11)61103-5