
Selective enrichment of TiO2 and precipitation behavior of perovskite phase in titania bearing slag
WANG Ming-yu(王明玉)1, ZHANG Lin-nan(张林楠)2, ZHANG Li(张 力)2,
SUI Zhi-tong(隋智通)2, TU Gan-feng(涂赣峰)2
1.School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2.School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China
Received 3 June 2005; accepted 28 November 2005
Abstract: The effects of additive agents and growth behavior of perovskite phase as well as temperature change of slag at semi industry scale test were studied. The results show that the increase of steel slag does good to titania enrichment, however, it isn’t useful for the growth and coarsening of the perovskite phase. The additive Si-Fe powder can promote titania enrichment and make perovskite phase grow up easily. While air is blown into the molten slag, the reduced components in slag are oxidized and the released heat raises the temperature of slag.
Key words: titania bearing slag; TiO2; selective enrichment; enrichment degree; perovskite phase; oxidizing
1 Introduction
China is rich in mineral resources of titanium, 92.4% of which is vanadium-titanium bearing magnetite, deposited mostly around the southwestern part. About 53% titanium is in the iron concentrate after mineral processing, after smelting process the blast furnace slag containing 20%-22% TiO2 were produced[1]. The content of TiO2 in the slag is too low to separate titanium dioxide, and it is also too high to produce slag cement[2]. This slag has been already accumulated 50 million tons so far, and it is still increasing with a rate of 3 million tons per year[3], resulting in a waste of titanium resources and environmental pollution. Several mineral processing and metallurgical processes[4, 5] have been proposed for treating the slag, such as middle grade rutile making, titanium pigment making by H2SO4 method and Si-Al-Ti alloy by smelting. However, due to the dispersed distribution of titanium component in various mineral phases, very fine grain size and complex interfacial combination, the application of these processes may result in poor recovery and high cost.
Fortunately, when the molten slag was carefully treated after it effused from blast furnace based on the selective separation technique[6, 7], most titania in slag could be enriched into a target mineral phase (perovskite) which then grew up. Therefore, the slag became the raw material for extracting titanium instead of a waste product. Both additive agents and oxidizing operation play an important role in the enrichment and growth of perovskite. LI et al[8, 9] studied the effect of CaO, MnO and Fe2O3 as additive on the precipitation temperature of perovskite phase. The effects of steel slag addition to titania bearing slag on viscosity and crystallization behavior of perovskite were investigated by LI et al[10]. LI et al[11] investigated the effect of basicity on enrichment selectivity of TiO2. DONG et al[12] forecasted the temperature change and heat releasing behavior of reductive slag during its oxidizing. Based on the research above, the effect of additive agents (steel slag and Si-Fe powder) and the temperature change in the oxidizing process at a pilot scale were studied in this work to provide a reference for compressively industrialized utilizing of those slags.
2 Experimental
The raw material was the bottom slag of blast furnace bearing about 20% TiO2. About 1.3 t molten slag mixed with additive agents (steel slag, Si-Fe powder) at different proportions was injected into a designed slag ladle and then a lance was used to blow air into the molten slag for 10-25 min. During this process, the temperature of slag was measured by disposable thermocouples. The slag obtained after these treatments was called treated slag, which was cooled slowly with the cooling rate of 1 K/min.
Slag samples were observed by metallographic microscope. Grain size distribution of perovskite and TiO2 enrichment degree (the enrichment degree defined by simple ratio of the mass per cent of titania in the enrichment phase to that whole titania in the slag) were determined by LETCAQ550/W 520 imagine analyzer. Enrichment degree and grain size distribution were measured as follows: 15 visual fields in each sample were stochasticly measured and then the statistical average was gotten as a result.
3 Results and discussion
3.1 Mineral components and selection of target phase of titania
It can be seen from Table 1[13] that though titania disseminates in various mineral phases in the slag, it mainly disperses in the perovskite and titannaugite phase. Due to TiO2 is the basal composition of perovskite and the high distribution ratio, so perovskite phase is selected as the target phase of titania; in addition, titannaugite phase is a finite solid solution which formed by 36.1% CaTiAl2O6 and diopside[14], i.e. titania in titannaugite phase can be turned into the perovskite phase by optimizing the treatment condition and appropriate additives, thereby achieving selective enrichment of TiO2.
Table 1 Distribution of TiO2 in titania bearing slag (mass fraction, %)
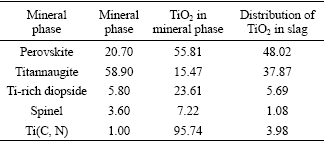
3.2 Enrichment behavior of TiO2
Fig.1 shows the relationship between the content of additives and the enrichment degree after cooling down in the designed slag ladle. It is obvious by comparison between the two curves that the enrichment degree is in connection with the additive agents’ content; the higher the additive agents are, the higher the enrichment degree is. While using steel slag as additive agent, the increase of TiO2 enrichment degree is more obvious than that of Si-Fe powder. The formation reaction of perovskite in the slag is as follows:
TiO2+CaO=CaTiO3(s) (1)
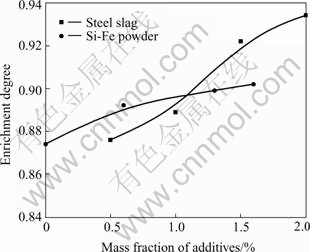
Fig.1 Relationship between additives and enrichment degree
It can be seen from Eqn.(1) that the increase of the activity of CaO and TiO2 can promote the forward reaction. Titania bearing slag was deoxidized slag, in which large numbers of low valency titanium ions such as Ti3+, Ti2+ existed. When air was blown into slag, the ions and Ti(C, N) could be oxidated into Ti4+ which made the activity of TiO2 increase, the reactions are as follows:
2TiO+O2=2TiO2 (2)
2Ti2O3+O2=4TiO2 (3)
2TiN(s)+2O2=2TiO2+N2 (4)
TiC(s)+2O2=TiO2+CO2 (5)
Furthermore, the increasing activity of CaO can also promote the reaction trend of perovskite formation. The addition of steel slag to titania bearing slag can cause the increase of CaO activity so that the enrichment degree increases. Chemical composition of additive agents is shown in Table 2.
Figs.2 and 3 show the relationship between temperature of molten slag and oxidizing time when Si-Fe powder and steel slag are chosen as the additive agents respectively. Obviously, the addition of Si-Fe powder can raise the temperature of slag remarkably during oxidizing process. Compared with steel slag as the additive agent, the rising temperature of molten slag is high, which shows that a great deal of heat will be released during Si-Fe powder oxidizing that resulted in the slag temperature going up. The higher the temperature is, the lower the viscosity of the molten slag is. As we know, perovskite is formed by the association reaction of Ca2+ and  , the decreasing viscosity can accelerate diffusion of Ca2+ and
, the decreasing viscosity can accelerate diffusion of Ca2+ and  , thus promotes precipitation of perovskite in the slag.
, thus promotes precipitation of perovskite in the slag.
Table 2 Chemical compositions of additive agents (mass fraction, %)


Fig.2 Curves of temperature change against time with Si-Fe as additive agent

Fig.3 Curves of temperature change against time with steel slag as additive agent
3.3 Morphology and grains distributions of perovskite in slag
Fig.4 shows the morphology of slag samples and corresponding grains distributions of perovskite phase. It can be clearly seen through the morphology that the perovskite grains of the treated slag are much bigger than that of the original slag. The area percent of perovskite phase in original slag is 13.9%, whose average grain size is larger than 30 ?m, but 63.6%, 74.6% and 92.5% in the treated slag respectively.
Fig.5 shows the relationship between the content of additive agents and the area percent of perovskite phase that grains are beyond 30 ?m. This figure shows clearly that with the increase of steel slag, the grains size of perovskite phase decreases, while grains size of perovskite phase increases with the increase of Si-Fe powder.
The initial oxidizing temperature of molten slag was about 1 350 ℃, which was far below the precipitating temperature(1 420 ℃)[15,16] of perovskite phase in slag, in other words, before oxidation a lot of perovskite crystal embryo had already existed. More heat would be released when Si-Fe powder was chosen as the additive agent, the temperature of slag could rise beyond 1 400 ℃ and such high temperature could hold about 15-20 min which decreased the precipitation content of perovskite phase in molten slag, then the original small crystal embryos of perovskite phase might melt down, only a few large ones could remain. The small number of nuclei of perovskite phase was in favor of grain growth and coarsening during the succeeding cooling process. Compared with Si-Fe powder as the additive agent, the lower the rising temperature, the shorter the high temperature duration, that is to say, the more amount of perovskite crystal embryos remained in slag, which resulted in perovskite grains average growing. Due to the approximately same grain size, it is difficult for the coarsing process through the growth of larger perovskite at the expense of smaller ones which show fine size and more perovskite grain particles in microstructure morphology. Through morphology of samples of treated slags shown in Figs.4(b), (b′), (d) and (d′), it can be clearly seen that the larger the perovskite grains, the smaller the perovskite grains amount than that of the slag with steel slag addition.
It is obvious by comparison between the two additive agents that the enrichment degree and the perovskite phase grain size are all correlative with their content during the oxidizing process. The more the content of steel slag, the higher the enrichment degree, but the smaller the grain size of the perovskite phase. On the other hand, the increase of steel slag can cause the viscosity of slag increase remarkably while the content beyond a certain extension, so it isn’t useful for the oxidizing farther. Although the increases of Si-Fe powder does good to the enrichment degree and perovskite phase grain size at the same time, the high cost limits its addition content. When the slag is comprehensively utilized at industry scale, steel slag and Si-Fe powder added at an appropriate proportion during oxidizing process can be taken into account.

Fig.4 Morphologies of slag((a)-(d)) and grain distributions of perovskite((a′)-(b′)): (a), (a′) Original slag without being oxidized; (b), (b′) Treated slag with 1.5% steel slag added; (c), (c′) Oxidized slag with no addition; (d), (d′) Treated slag with 1.6% Si-Fe powder added

Fig.5 Relationship between additive and area percent
4 Conclusions
From the experiment, oxidizing and appropriate additive agents is an effective technique for treating titania bearing slag. Under the experimental condition, TiO2 can be easily enriched and perovskite phase can also grow up remarkably.
1) The more the content of steel slag addition in titana bearing slag is, the higher the titania enrichment degree is, however, the more the addition is, the smaller the grain size of the perovskite phase is.
2) When the content of Si-Fe powder increases, the titania enrichment degree increases and perovskite phase grain size grows up.
3) A great deal of heat will be released during slag oxidizing, which can result in the slag temperature going up whether additive agent is added or not.
References
[1] MA Jun-wei, SUI zhi-tong, CHEN Bing-chen. Separating titania from treated slag by gravity separation or flotation [J]. Trans Nonferrous Met Soc China, 2000, 10(4): 520-523.
[2] WANG Xi-qing. The Smelting of Vanadium-Titanium Bearing Magnetite by Blast Furnace[M]. Beijing: Metallurgical Industry Press, 1994. 68-70.
[3] HUANG Zhen-qi, WANG Ming-hua, DU Xing-hong, SUI Zhi-tong. Recovery of titanium from the rich titanium slag by H2SO4 method[J]. J Mater Sci Technol, 2003, 19(2): 191-192.
[4] WANG ming-yu, LIU Xiao-hua, SUI Zhi-tong. The comprehensive utilization of smelting slag[J]. Multipurpose Utilization of Mineral Resources, 2003(3): 28-32.
[5] PENG Bing, YI Wen-zhi, PENG Ji, YU Di. A kind of way for comprehensive utilization of Panzhihua blast furnace slags[J]. Multipurpose Utilization of Mineral Resources, 1997(6): 26-30.
[6] SUI Zhi-tong, ZHANG Pei-xin, Yamauchi C. Precipitation selectivity of boron compounds from slags[J]. Acta Mater, 1999, 47(4): 1337-1344.
[7] SUI Zhi-tong, ZHANG Li, LOU Tai-ping, et al. Green separation technique of valuable metals from metallurgical slags[J]. Metal Mine(Supplement), 2003: 356-358.
[8] LI Yu-hai, LOU Tai-ping, SUI Zhi-tong. Selective enrichment of Ti component in Ti-bearing blast furnace slag and precipitation behavior of perovskite phase[J]. The Chinese Journal of Nonferrous Metals, 2000, 10(5): 719-722.(in Chinese)
[9] LI Yu-hai, LOU Tai-ping, SUI Zhi-tong. Effects of CaO and MnO on the crystallization of the perovskite phase in the Ti-bearing blast furnace slag[J]. Journal of Iron and Steel Research, 2000, 12(3): 1-4.
[10] LI Yu-hai, LOU Tai-ping, SUI Zhi-tong. The effects of steel slag on the viscosity of titanium-bearing blast furnace slag and crystallization behavior of perovskite[J]. Journal of Shenyang Institute of Technology, 2002, 21(2): 6-9.
[11] LI Liao-shao, SUI Zhi-tong. Physical chemistry behavior of enrichment selectivity of TiO2 in perovskite[J]. Acta Phys Chim Sin, 2001, 17(9): 845-849.
[12] DONG Yuan-chi, LI LIAO-sha, SUI Zhi-tong. The cooling and heat releasing behavior of reductive slag during its oxidizing[A]. Proceedings of the TMS Fall Extraction and Processing Conference[C]. Charalotte: Mineral, Metals and Materials Society, 2004. 613-621.
[13] LI Ying-tang. Application Mineralogy [M], Beijing: Science Press, 1995. 296.
[14] WU Ben-xian. Panzhihua Vanadium Titanium Magnetite Processing Mineralogy[M]. Chengdu: Sichuan Science and Technology Press, 1998. 151.
[15] LOU Tai-ping, LI Yu-hai, LI liao-sha, SUI Zhi-tong. Study on kinectics of perovskite phase precipitate in slag bearing titanium [J]. Journal of the Chinese Ceramic Society, 2000, 28(3): 255-258.
[16] LI Ying, LIU Cheng-jun, JIANG Mao-fa, CHENG Nai-liang, ZOU Jun-su. Physico-chemical properties of titania slag during cooling process [J]. Journal of Iron and Steel Research, 2004, 16(3): 68-70.
Foundation item: Project(50234040) supported by the National Natural Science Foundation of China
Corresponding author: WANG Ming-yu; Tel: +76-731-8830143; E-mail: wmydxx@sohu.com
(Edited by LI Xiang-qun)