A new low-bandgap polymer acceptor based on benzotriazole for efficient all-polymer solar cells
来源期刊:中南大学学报(英文版)2021年第7期
论文作者:邹应萍 李哲 陈泓钢 袁俊 邹洁 李静 管慧兰
文章页码:1919 - 1931
Key words:all-polymer solar cells; polymer acceptor; low-bandgap; benzotriazole; power conversion efficiency
Abstract: The rational design of polymer acceptors with strong and broad absorption is critical to improve photovoltaic performance. In this work, a new polymer acceptor PY9-T based on heptacyclic benzotriazole (Y9-C16) as a building block and thiophene unit as the linking unit was synthesized, which exhibited a low bandgap (1.37 eV) and a high extinction coefficient of the neat film (1.44×105 cm-1). When PY9-T was blended with the wide bandgap polymer donor PBDB-T, the all-polymer solar cells (APSCs) showed a high power conversion efficiency (PCE) of 10.45% with both high open circuit voltage of 0.881 V and short-circuit current density of 19.82 mA/cm2. In addition, APSCs based on PY9-T show good thermal stability, as evidenced by slight changes morphologies when annealed at 100 ℃. These results suggest that Y9-C16 provides a new building block to develop efficient and stable polymer acceptors.
Cite this article as: LI Zhe, CHEN Hong-gang, YUAN Jun, ZOU Jie, LI Jing, GUAN Hui-lan, ZOU Ying-ping. A new low-bandgap polymer acceptor based on benzotriazole for efficient all-polymer solar cells [J]. Journal of Central South University, 2021, 28(7): 1919-1931. DOI: https://doi.org/10.1007/s11771-021-4741-7.

J. Cent. South Univ. (2021) 28: 1919-1931
DOI: https://doi.org/10.1007/s11771-021-4741-7
LI Zhe(李哲), CHEN Hong-gang(陈泓钢), YUAN Jun(袁俊), ZOU Jie(邹洁),LI Jing(李静), GUAN Hui-lan(管慧兰), ZOU Ying-ping(邹应萍)
College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract: The rational design of polymer acceptors with strong and broad absorption is critical to improve photovoltaic performance. In this work, a new polymer acceptor PY9-T based on heptacyclic benzotriazole (Y9-C16) as a building block and thiophene unit as the linking unit was synthesized, which exhibited a low bandgap (1.37 eV) and a high extinction coefficient of the neat film (1.44×105 cm-1). When PY9-T was blended with the wide bandgap polymer donor PBDB-T, the all-polymer solar cells (APSCs) showed a high power conversion efficiency (PCE) of 10.45% with both high open circuit voltage of 0.881 V and short-circuit current density of 19.82 mA/cm2. In addition, APSCs based on PY9-T show good thermal stability, as evidenced by slight changes morphologies when annealed at 100 ℃. These results suggest that Y9-C16 provides a new building block to develop efficient and stable polymer acceptors.
Key words: all-polymer solar cells; polymer acceptor; low-bandgap; benzotriazole; power conversion efficiency
Cite this article as: LI Zhe, CHEN Hong-gang, YUAN Jun, ZOU Jie, LI Jing, GUAN Hui-lan, ZOU Ying-ping. A new low-bandgap polymer acceptor based on benzotriazole for efficient all-polymer solar cells [J]. Journal of Central South University, 2021, 28(7): 1919-1931. DOI: https://doi.org/10.1007/s11771-021-4741-7.
1 Introduction
Bulk-heterojunction polymer solar cells (BHJ-PSCs) have been regarded as one of the prospective photovoltaic technologies due to the merits of simple device structure, light weight, flexibility, and large-area printed electronics [1-4]. Among the PSCs, all-polymer solar cells based on a blend of polymer donor and polymer acceptor offer unique advantages such as robustness of film morphology, stability of the mechanical stresses, and compatibility of large scale manufacturing [5-8]. Recently, the rapid development of small molecule acceptors (SMAs) has achieved a power conversion efficiency (PCE) of 17.5% (certified) for single-junction devices while the development of polymer acceptors still lags behind. Therefore, developing high-performance polymer acceptor materials is crucial to boost the photovoltaic performance of APSCs.
Many polymer acceptor materials are applied to all-polymer solar cells (APSCs). For example, poly{[N,N`-bis(2-octyldodecyl)-naphthalene-1, 4, 5, 8-bis(dicarboximide)-2,6-diyl]-alt-5,5`-(2,2`-
bithiophene)} (N2200) has demonstrated efficient photovoltaic performance. However, its low absorption coefficient limits the photocurrent and PCE of APSCs [9-11]. Hence, it is necessary to develop new polymer acceptors with strong absorption in visible and near-infrared (vis-NIR) regions and high absorption coefficient. ZHANG et al [5] reported a new design strategy to increase the absorption coefficient of polymer acceptor by using fused-ring SMAs (IDIC-C16) as a building block and thiophene as the linking unit. The corresponding polymer acceptor PZ1 showed a high PCE of 9.19%. Based on this strategy, different types of polymer acceptors are constructed [6, 12-15]. For example, YAO et al [13] reported a new polymer acceptor PFBDT-IDTIC with a fluorinated benzodithiophene (BDT) unit achieving the PCE of 10.3%. Hence, the rational design of materials based on fused ring SMA is an efficient strategy to improve photovoltaic performance.
Recently, “Y-series” non-fullerene acceptors were reported, such as Y6, with high efficiency [16-22]. These acceptor materials have many advantages such as strong absorption in the vis-NIR region, low voltage loss, and high electron mobility. Therefore, reasonable selection of “Y-series” SMAs as building blocks is significant to improve performance. For example, JIA et al [23] chose the derivative of Y6 as building block to prepare the polymer acceptor PJ1 obtaining a PCE of 14.4%. In this work, we designed and synthesized a novel polymer acceptor PY9-T based on a thiophene unit and a SMA of Y9-C16 with a similar molecular backbone to the acceptor Y9 [24]. The PY9-T not only has advantages of Y9 acceptor (e.g., strong absorption in the 500-900 nm region), but merits of
conjugated polymers (e.g., large π-electron delocalization) [25]. The PY9-T exhibits a low-bandgap and a relatively high lowest unoccupied molecular orbital (LUMO) level. Furthermore, blended with wide-bandgap polymer donor PBDB-T (Figure 1(a)), the PY9-T-based device achieved a high PCE of 10.45%. The results indicate that PY9-T is a potential polymer acceptor for efficient APSCs.
2 Results and discussion
2.1 Synthesis and characterization
The synthesis of PY9-T began with compound 1 (Figure S1). Compound 2 was synthesized through the Cadogan reaction of compound 1 and then nucleophilic substitution reaction to introduce the large 2-hexyldecadecyl branched chains. The aldehyde group was introduced into the fused-ring thiophene unit by Vilsmeier-Haack reaction to give compound 3. Y9-C16-Br was obtained by Knoevenagel condensation reaction of compound 3 and IC-Br. The polymer PY9-T (Figure 1(b)) was synthesized by a Stille coupling reaction between Y9-C16-Br and 2,5-bis(trimethylstannyl)thiophene, exhibiting good solubility in organic solvents (e.g., chloroform and chlorobenzene). These synthetic details of Y9-C16-Br monomer, polymerization, and their nuclear magnetic resonance (NMR) spectra are displayed in experimental section in Figures S1-S6. Herein, the PY9-T shows the average molecular weight (Mw) of 1.24 kDa and a polydispersity index (PDI) of 1.9, measured by gel permeation chromatography (GPC) with1,2-dichlorobenzene as the eluent under at 140 °C (Figure S7). Thermal gravimetric analyzer (TGA) and differential scanning calorimetry (DSC) were used to measure the thermal stability of PY9-T (Figure S8). TGA exhibits a decomposition temperature (5% weight loss) of 344 °C under N2 atmosphere and DSC shows negligible melting phase transition in the temperature range of 20-300 °C, indicating good thermal stability.

Figure 1 (a) Chemical structure of PBDB-T; (b) Chemical structure of PY9-T; (c) Normalized UV-vis absorption spectra of PBDB-T and PY9-T in neat films and PY9-T in chloroform solution; (d) Absorption coefficient of PY9-T and PBDB-T in films; (e) Energy level diagram of PBDB-T and PY9-T; (f) Cyclic voltammograms of PY9-T
2.2 Photophysical and electrochemical properties
The ultraviolet-visible absorption of PBDB-T and PY9-T is shown in Figure 1(c). The film absorption of PY9-T can be observed in the range of 500-900 nm with a maximum peak at 821 nm and two weak shoulder peaks at 654 and 744 nm. Meanwhile, the film absorption of PBDB-T shows complementary absorption in the range of 350-700 nm with a maximum peak at 624 nm. Compared with the diluted solution, the film of PY9-T shows a 23 nm redshift of the maximum absorption peak, which indicates more strong aggregations in the film. The optical bandgap (Egopt=1240/λonset) of PY9-T deduced from the absorption onset wavelengths (λonset) is 1.37 eV and the absorption coefficient of PY9-T is 1.44×105 cm-1 (Figure 1(d)), which is about five times higher than N2200 (0.3×105 cm-1) [5]. The low bandgap and complementary absorption are beneficial to light-harvesting to ensure the higher short-circuit current density (Jsc) for PBDT-T: PY9-T based devices. The electrochemical energy levels of PY9-T were measured by cyclic voltammetry (CV) method. The highest occupied molecular orbital (HOMO) and LUMO energy levels were calculated from the onset oxidation potential (Eox) and the onset reduction potential (Ered) via the equation of EHOMO/LUMO=-(Eox/red+4.8-EFc/Fc+) (eV), where EFc/Fc+ was carried out to be 0.44 eV, and Fc is ferrocene. The HOMO and LUMO levels of PY9-T are -5.56 and -3.72 eV, respectively (Figures 1(e) and (f)). Generally, the higher LUMO energy level of PY9-T is conducive to the larger open circuit voltage (Voc) [26, 27]. Corresponding optical
properties and energy levels of PY9-T are listed in Table S1.
2.3 Photovoltaic properties
To further investigate the photovoltaic performance of the PY9-T, the conventional device structure with ITO(indium tin oxide)/PEDOT:
PSS (poly(3,4-ethylenedioxythiophene):poly-(styrenesulfonate))/PBDB-T:PY9-T /PDINN (2,9-bis(3-((3-(dimethylamino)propyl)amino)propyl) anthrax [2,1,9-def:6,5,10-def]diisoquinoline-1,3,8,10(2H,9H)-tetraone)/Ag was fabricated. The
current density-voltage (J-V) curves were measured and the photovoltaic data are shown in Figure 2(a) and Table 1, respectively. Different weight ratios of PBDB-T: PY9-T were tried and the optimal weight ratio was 1:1. In addition, thermal annealing (TA) at different temperatures was carried out, which increases fill factor (FF) with a slight effect on Jsc and Voc. Furthermore, ratios of 1-chloronaphthalene (CN) additive were performed to optimize the device performance. As a result, the optimal PCE (10.45%) was obtained under the conditions of PBDB-T: PY9-T mass ratio of 1:1, TA at 100 °C for 10 min and 5% CN additive (Table S2-5). The statistical distribution of the PCE of 15 devices (Figure 2(c)) in optimal conditions is further presented to demonstrate the good reproducibility of PY9-T-based all-PSCs. Here, the energy loss (Eloss) was estimated by using the difference between the Egopt of the acceptor and the energy of the extracted charges (qVoc) of the device (Eloss=Egopt-qVoc). And the optimized APSC exhibited the low Eloss of 0.49 eV [25, 28].
The external quantum efficiency (EQE) spectra of the PBDB-T: PY9-T based-APSCs is shown in Figure 2(b). Compared with the as-cast device, the EQE values of the optimized devices were higher in the range of 430-870 nm, which indicated more efficient photoelectron conversion and matched well with the enhanced Jsc of the optimized devices. The Jsc values of as-cast and optimized devices integrated from the EQE curves were 18.38 and 19.54 mA/cm2, which agreed well with those from the J-V measurements within 5% errors.
Table 1 Photovoltaic parameters data based on PBDB-T:PY9-T blend films under different conditions

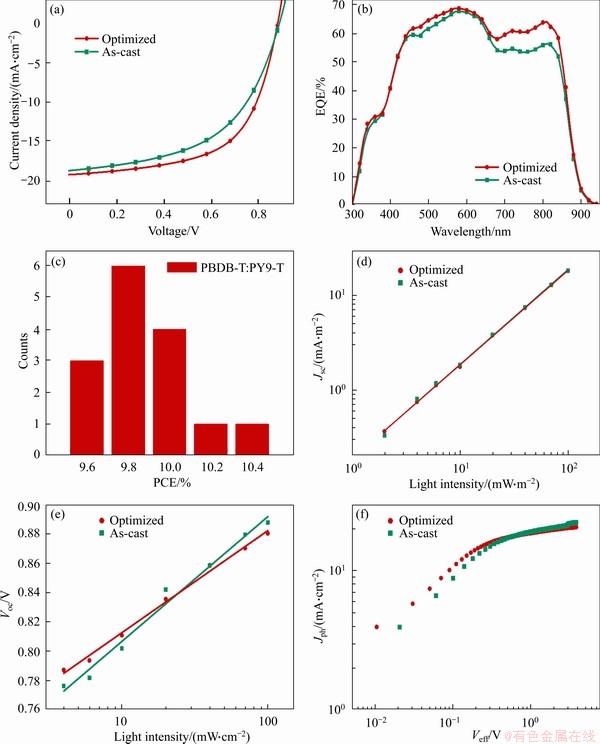
Figure 2 J-V curves (a), EQE spectra (b), bar graphs of PCE counts for 15 individual devices (c), Jsc dependence upon different Plight (d), Voc dependence upon different Plight (e) and Jph-Veff curves of corresponding APSCs based on PBDB-T: PY9-T (f)
2.4 Charge transport properties
The charge carrier mobilities of the PBDB-T: PY9-T film with optimized and as-cast devices were evaluated by fabricating the hole-only device ITO/PEDOT:PSS/PBDB-T:PY9-T/MoO3/Ag and electron-only device ITO/ZnO/PBDB-T:PY9-T/ PDINN/Ag by using the space charge limited current (SCLC) method (Figure S9). For the as-cast device, electron mobilities (μe) and hole mobilities (μh) are calculated as 0.883×10-4 and 0.816×10-4 cm2/(V·s), respectively, with μe/μh of 1.08. By comparison, higher and more balanced values with μe and μh of 1.62×10-4 and 1.64×10-4 cm2/(V·s) (μe/μh of 0.99) are achieved after optimization, which can lead to notably increased FF and Jsc for the optimized devices.
2.5 Exciton dissociation and charge recombination
The exciton dissociation and recombination behaviors have significant influence on Jsc and FF. Generally, Jsc and Plight follow a power law with the relationship Jsc∝Plightα (Plight is the light intensity and α is a power-law component) [29]. Experimentally, α approaching 1 indicates that the bimolecular recombination is negligible. The α values for the optimized and as-cast devices are 1.006 and 0.999, respectively, implying efficient exciton dissociation and weaker bimolecular recombination in APSCs based on PY9-T (Figure 2(d)). Besides, the Voc versus Plight (Figure 2(e)) is determined as the formula of Voc∝(nkT/q)ln(Plight), where n is a constant, K is the Boltzmann constant, T is the temperature and q is the elementary charge. After optimization, the value of slope decreased from 1.48 to 1.21 kT/q, which suggested a lower trap state [30]. The photocurrent (Jph) and the effective voltage (Veff) of the active layer were also carried out to study exciton dissociation probabilities (Pdiss); Jph is evaluated by the formula Jph=JL-JD, where JL and JD are the current densities under illumination and darkness conditions, respectively; Veff expresses as Veff=V0-Vbias, where V0 is the voltage when Jph=0, and Vbias is the applied bias [31]. When Veff is higher than 2 V, Jph will reach the saturation current density (Jsat) (Figure 2(f)). Besides, the Pdiss follows the formula Pdiss=Jph/Jsat. The Pdiss of the as-cast and optimized devices are 91.7% and 93.2% respectively, indicating a high exciton generation and dissociation efficiency.
2.6 Thermal stability and morphology characterization
The thermal stability of APSCs based on PBDT-T: PY9-T was investigated by increasing the TA time at 100 °C, as shown in Figure 10. The device maintains an 86% PCE of the initial efficiency with Voc of 0.868 V, Jsc of 19.18 mA/cm2, and FF of 54.20% after being annealed for 180 min (PCE=9.02%). Atomic force microscopy (AFM) and transmission electron microscopy (TEM) further confirmed these results. As shown in Figures 3(a)-(c), after annealing films for various times, the devices obtain interpenetrating network structure with almost identical root-mean-square surface roughness (Rq) of around 1.40 nm. In addition, fine nanoscale phase separation is also retained in TEM when the active layer is annealed for 180 min (Figures 3(d)-(f)). The results demonstrate that APSC based on PY9-T has potential as a candidate for morphological stable PSCs.
3 Conclusions
A new polymer acceptor PY9-T with benzotriazole fused-ring acceptor as the building block is designed and synthesized. It exhibits strong and broad absorption in 500-900 nm with a high extinction coefficient (1.44×105 cm-1). When PY9-T was matched with the polymer donor PBDB-T, the devices based on PBDB-T: PY9-T achieved the PCE of 10.45%. PY9-T-based APSCs show good thermal stability owing to no apparent change in morphology. These findings demonstrate that PY9-T is a promising polymer acceptor candidate for high-performance and thermal stable APSCs.

Figure 3 AFM (a-c) and TEM (d-f) images for PBDB-T: PY9-T films with various TA times:
Supporting information
1 Basic characterization
1H NMR and 13C NMR spectra were recorded using a Bruker AV-500 spectrometer in deuterated chloroform solution at 298 K, unless specified otherwise. Chemical shifts were reported as δ values (10-6) with tetramethylsilane (TMS) as the internal reference. UV-Vis absorption spectra were recorded on the SHIMADZU UV-2600 spectrophotometer. For the solid state measurements, PBDB-T and PY9-T solutions in chloroform were spin-coated on quartz plates. The cyclic voltammetry was recorded with a computer controlled CHI 660E electrochemical workstation using PBDB-T or BZIC films on platinum electrode (1.0 cm2) as the working electrode, a platinum wire as the counter electrode and Ag/AgCl (0.1 mol/L) as the reference electrode in an anhydrous and argon-saturated solution of 0.1 mol/L of tetrabutylammonium hexafluorophosphate (Bu4NPF6) in anhydrous acetonitrile at a scanning rate of 50 mV/s. Electrochemical onsets were determined at the position where the current starts to differ from the baseline. The morphologies of the PBDB-T/PY9-T blend films were investigated by atomic force microscopy (AFM, Agilent Technologies,5500 AFM/SPM System, USA).
2 Fabrication and characterization of all-polymer solar cells
The devices were fabricated with an indium tin oxide (ITO) glass as positive electrode and a Ag as negative electrode. Patterned ITO glass with a sheet resistance of 15 Ω was purchased from CSG HOLDING Co. Ltd. (China). The ITO glass was cleaned by sequential ultrasonic treatment in detergent, deionized water, acetone and isopropanol, and then treated in an ultraviolet-ozone chamber (Ultraviolet Ozone Cleaner, Jelight Company, USA) for 20 min. Then PEDOT: PSS (poly(3,4-ethylene dioxythiophene): poly(styrenesulfonate)) (Baytron PVP Al 4083, Germany) was filtered through a 0.45 μm poly(tetrafluoroethylene) (PTFE) filter and spin coated at 6000 r/min for 30 s on the ITO substrate. Subsequently, PEDOT: PSS film was baked at 150 °C for 15 min in the air, and the thickness of the PEDOT:PSS layer is about 30 nm. The polymer PBDB-T and PY9-T (13 mg/mL) were dissolved in CHCl3 and different volume ratios of 1-chloronaphthalene (CN) (Sigma Aldrich) overnight and spin-cast at 2800 r/min for 30 s onto the PEDOT: PSS layer. After spin-coating, the active layers were annealed at different temperature for various time. The thickness of the active layers is ca. 100 nm. Then methanol solution of PDINN at a concentration of 1.0 mg/mL was deposited atop the active layer at 3000 r/min for 30 s to afford a PDINN cathode buffer layer with thickness of ca. 10 nm. Finally, top Ag electrode was deposited in vacuum onto the cathode buffer layer at a pressure of ca. 1.0×10-5 Pa. The active area of the devices was 6 mm2. Device characterization was carried out under AM 1.5G irradiation with the intensity of 100 mW/cm2 (Oriel 67005, 500 W), calibrating by a standard silicon cell. J-V curves were recorded with a Keithley 236 digital source meter. An xenon lamp with AM 1.5 filter was used as the white light source and the optical power was 100 mW/cm2. The EQE measurements of organic solar cells were performed by solar cell spectral response measurement system QE-R3-011 (Enli Technology Co., Ltd., Taiwan, China). A calibrated silicon detector was used to determine the absolute photosensitivity at different wavelengths. In addition to EQE, all of these fabrications and characterizations were conducted in a glove box.
3 Materials and synthesis
Tetrakis(triphenylphosphine)palladium
(Pd(PPh3)4), bis(triphenyl-phosphine)palladium(II) dichloride(PdCl2(PPh3)2) and n-butyllithium (n-BuLi) were obtained from J&K Energy Chemical Company, China, and they were all used as received. PBDB-T was purchased from Solarmer Energy Inc. IC-Br was obtained from SunaTech Inc. All other reagents and solvents were purchased commercially as analytical pure and used without further purification. 2-(2-ethylhexyl)-5, 6-dinitro-4,7-bis(6-undecylthieno[3,2-b]thiophen-2-yl)-2H-benzo[d] [1, 2, 3] triazole (1) was synthesized according to previous literatures [24].
6-(2-ethylhexyl)-12,13-bis(2-hexyldecyl)-3,
9-diundecyl-12,13-dihydro-6H-thieno[2'',3'':4',5']
thieno[2',3':4,5]pyrrolo[3,2-g]thieno[2',3':4,5]thieno
[3,2-b][1,2,3]triazolo[4,5-e]indole (2). Compound 1 (2.00 g, 2.21 mmol) and triphenylphosphorus (5.787 g, 22.07 mmol) were dissolved in the anhydrous N-Methyl pyrrolidone (NMP, 50 mL) into a 250 mL three-necked round bottom flask under argon. The reation was heated at 180 °C overnight. After cooling to room temperature, the brown solution was added 7-(bromomethyl)pentadecane (4.10 g, 13.24 mmol), potassium hydroxide (1.24 g, 22.07 mmol), potassium iodide (0.15 g, 0.88 mmol) at room temperature and the mixture with atmosphere was refluxed at 90 °C for 15 h. The residue was extracted with dichloromethane (DCM) and H2O three times. The organic layers were combined and dried over MgSO4, and purified with column chromatography on silica gel using DCM/petroleum ether (PE) (1/8, v/v) as the eluent to give a brownish red liquid 2 (1.06 g, 37.09 % yield). 1H NMR (400 MHz, CDCl3) δH 6.87 (s, 2H), 4.64 (d, J=7.2 Hz, 2H), 4.49 (d, J=7.6 Hz, 4H), 2.72 (t, J=7.6 Hz, 4H), 2.35-2.22 (m, 1H), 1.97-1.88 (m, 2H), 1.80-1.73 (m, 4H), 1.38-1.16 (m, 44H), 0.94-0.87 (m, 24H), 0.80-0.72 (m, 32H), 0.60 (t, J=6.8 Hz, 12H).
Synthesis of 6-(2-ethylhexyl)-12,13-bis(2-
hexyldecyl)-3,9-diundecyl--12,13-dihydro-6H-
thieno[2'',3'':4',5']thieno[2',3':4,5]pyrrolo[3,2-g]
thieno[2',3':4,5]thieno[3,2-b][1,2,3]triazolo[4,5-e]
indole-2,10-dicarbaldehyde (3). To a solution of compound 2 (0.72 g, 0.56 mmol) with 1,2-dichloroethane (20 mL) into a 100 mL three-necked round bottom flask under argon and anhydrous N,N-dimethyl-formamide (DMF)(1.7 mL) was added at once. Then, POCl3 (0.9 mL) was added dropwise slowly at 0 °C for 2 h; the mixture was heated to 90 °C and stirred overnight.
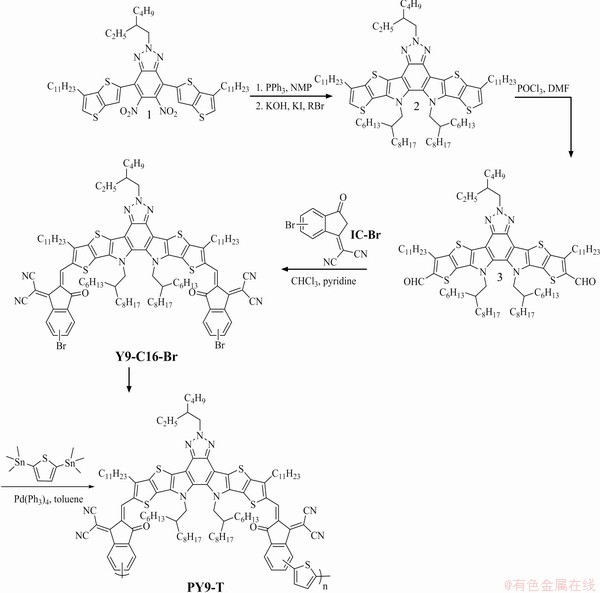
Figure S1 Synthetic routes of PY9-T
The reaction mixture was poured into water (200 mL), and then extracted with DCM-H2O three times. The combined organic layer was evaporated under reduced pressure and the crude product was purified with column chromatography on silica gel using DCM/PE(1/1, v/v) as the eluent to give a orange red liquid 3 (0.40 g, 53.11 % yield). 1H NMR (400 MHz, CDCl3) δH 10.05 (s, 2H), 4.65 (d, J=7.2 Hz, 2H), 4.53 (d, J= 7.6 Hz, 4H), 3.11 (t, J=7.2 Hz, 4H), 2.30-2.27 (m, 1H), 1.88-1.81 (m, 6H), 1.40-1.19 (m, 44H), 0.99-0.89 (m, 24H), 0.82-0.73 (m, 32H), 0.60 (t, J=7.2 Hz, 12H).
Synthesis of Y9-C16-Br. Compound 3 (0.39 g, 0.29 mmol), compound IC-Br (0.24 g, 0.88 mmol), pyridine (1 mL) and chloroform (30 mL) were dissolved in a round bottom flask under argon. The mixture was stirred at 65 °C overnight. After cooling to room temperature, the mixture was poured into methanol and filtered. The residue was purified with column chromatography on silica gel using DCM/PE (1/1, v/v) as the eluent to give a blue black solid (0.37 g, 68.04% yield). 1H NMR (400 MHz, CDCl3) δH 9.15 (s, 2H), 8.82 (d, J=0.8 Hz, 1.27H), 8.53 (d, J=9.2 Hz, 0.70H), 8.01 (d, J=1.6 Hz, 0.63H), 7.88-7.82 (m, 1.70H), 7.80-8.76 (m, 1.71H), 4.74-4.73 (m, 6H), 3.21 (t, J=8.0 Hz, 4H), 2.40-2.34 (m, 1H), 2.08-2.05 (m, 2H), 1.91-1.84 (m, 4H), 1.56- 1.28 (m, 44H), 1.14-0.85 (m, 56H), 0.79 (t, J=7.2 Hz, 12H). 13C NMR (CDCl3, 400 MHz): dC 187.30, 186.88, 160.07, 159.48, 155.95, 153.69, 149.19, 144.99, 141.43, 138.55, 138.36, 137.96, 137.86, 137.42, 137.01, 136.65, 135.93, 135.49, 133.74, 133.49, 129.97, 129.91, 129.24, 128.09, 126.58, 124.41, 119.70, 119.61, 115.32, 114.76, 112.01, 99.28, 68.25, 67.75, 59.72, 55.44, 40.38, 39.13, 31.92, 31.84, 31.62, 31.57, 31.28, 30.59, 30.49, 29.90, 29.84, 29.76, 29.67, 29.62, 29.53, 29.50, 29.41, 29.35, 29.21, 28.41, 25.51, 23.99, 22.95, 22.69, 22.62, 22.50, 14.12, 14.06, 14.03, 10.51.
4 Mobility measurements
Hole and electron mobilities were measured using the space charge limited current (SCLC) method, with the hole-only device of ITO/PEDOT:PSS/PBDB-T:PY9-T/MoO3/Ag and electron-only device for hole mobility measurement and electron-only device of ITO/ZnO/PBDB-T:PY9-T/PDINN/Ag for electron mobility measurement. The SCLC mobilities were calculated by MOTT-Gurney equation [32, 33]:
 (1)
(1)
where J is the current density; εr is the relative dieletiric constant of active layer material usually 2-4 for organic semiconductor, herein we use a relative dielectric constant of 4; ε0 is the permittivity of empty space; μ is the mobility of hole or electron and L is the thickness of the active layer; V is the internal voltage in the device, and V=Vapp-Vbi, where Vapp is the voltage applied to the device, and Vbi is the built-in voltage resulting from the relative work function difference between the two electrodes (in the hole-only and the electron-only devices, and the Vbi values are 0.2 and 0 V, respectively).
5 Additional figures and tables
Table S1 Optical properties and energy levels of PY9-T and PBDB-T

Table S2 Photovoltaic data of all-PSCs based on PBDB-T:PY9-T blend films at different D/A weight ratios with 0.5% CN additive treatment and TA at 100 °C for 10 min, under irridiation of AM 1.5G, 100 mW/cm2

Table S3 Photovoltaic performance parameters of all-PSCs based on PBDB-T:PY9-T (1/1) at different concentration of additive (vol%) with TA at 100 °C for 10 min, under irradiation of AM 1.5G, 100 mW/cm2

Table S4 Photovoltaic performance parameters of all-PSCs based on PBDB-T:PY9-T (1/1) at different temperatures of TA for 10 min with 0.5% CN additive treatment, under irridiation of AM 1.5G, 100 mW/cm2

Table S5 Photovoltaic performance parameters of all-PSCs based on PBDB-T:PY9-T (1/1) at different times of TA 100 °C with 0.5% CN additive treatment, under irradiation of AM 1.5G, 100 mW/cm2

Table S6 Abbreviations of structures

Figure S2 1H-NMR spectrum of compound 2 in CDCl3

Figure S3 1H-NMR spectrum of compound 3 in CDCl3

Figure S4 1H-NMR spectrum of compound Y9-C16-Br in CDCl3

Figure S5 1H-NMR spectrum of compound PY9-T in CDCl3
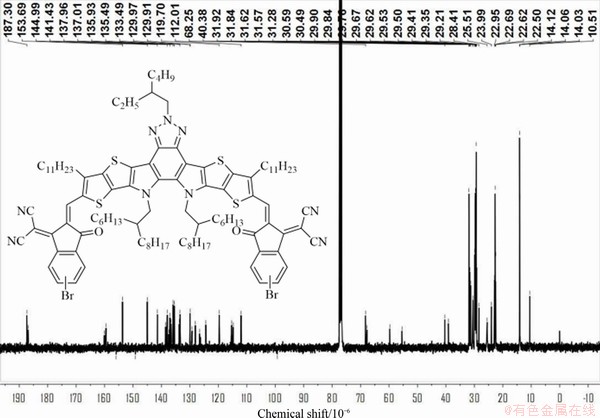
Figure S6 13C-NMR spectrum of compound Y9-C16-Br in CDCl3

Figure S7 GPC measurement of PY9-T

Figure S8 TGA curve (a) and DSC thermogram (b) of PY9-T

Figure S9 J1/2-V characteristics of (a) electron only devices and (b) hole only devices. Solid lines are fitting lines of the data
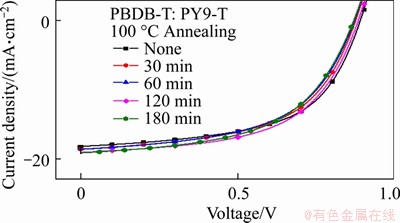
Figure S10 J-V curves of corresponding APSCs with 100 °C annealing for different time
Contributors
LI Zhe, YUAN Jun and ZOU Ying-ping conceived and directed the experiments. LI Zhe, ZOU Jie, GUAN Hui-lan and LI Jing performed the material synthesis. CHEN Hong-gang performed fabrication of devices. The first draft was written by LI Zhe, YUAN Jun and ZOU Ying-ping revised and edited the manuscript. ZOU Ying-ping supervised this work.
Conflict of interest
LI Zhe, CHEN Hong-gang, YUAN Jun, ZOU Jie, LI Jing, GUAN Hui-lan, and ZOU Ying-ping declare that they have no conflict of interest.
References
[1] YU Gang, GAO J, HUMMELEN J C, WUDI F, HEEGER A J. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions [J]. Science, 1995, 270(5243): 1789-1791. DOI: 10.1126/ science.270.5243.1789.
[2] HALLS J J M, PICHLER K, FRIEND R H, MORATTI S C, HOLMES A B. Exciton dissociation at a poly (p-phenylenevinylene)/CeO heterojunction [J]. Synthetic Metals, 1996, 77(1-3): 277-280. DOI: 10.1016/0379-6779(96)80102-0.
[3] LI Yong-fang, ZOU Ying-ping. Conjugated polymer photovoltaic materials with broad absorption band and high charge carrier mobility [J]. Advanced Materials, 2008, 20(15): 2952-2958. DOI: 10.1002/adma.200800606.
[4] LI Chen, LIU Miao-yin, PSCHIRER N G, BAUMGARTEN M, MULLEN K. Polyphenylene-based materials for organic photovoltaics [J]. Chemical Reviews, 2010, 110(11): 6817-6856. DOI: 10.1021/cr100052z.
[5] ZHANG Zhi-guo, YANG Yan-kang, YAO Jia, XUE Ling-wei, CHEN Shan-shan, LI Xiao-jun, MORRISON W, YANG Chang-duk, LI Yong-fang. Constructing a strongly absorbing low-bandgap polymer acceptor for high-performance all-polymer solar cells [J]. Angewandte Chemie International Edition, 2017, 56: 13503-13507. DOI: 10.1002/anie. 201707678.
[6] ZHANG Zhi-guo, LI Yong-fang. Polymerized small molecule acceptors for high performance all-polymer solar cells [J]. Angewandte Chemie International Edition, 2020, 60(9): 2-14. DOI: 10.1002/anie.202009666.
[7] WANG Gang, MELKONYAN F S, FACCHETTI A, MARKS T J. All-polymer solar cells: Recent progress, challenges, and prospects [J]. Angewandte Chemie International Edition, 2019, 58(13): 4129-4142. DOI: 10.1002/anie.201808976.
[8] DUAN Chun-hui, DING Li-ming. The new era for organic solar cells: Polymer donors [J]. Science Bulletin, 2020, 65(17): 1422-1424. DOI: 10.1016/j.scib.2020.04.044.
[9] LI Zhao-jun, XU Xiao-feng, ZHANG Wei, MENG Xiang-yi, MA Wei, YARTSEV A, INGANAS O, ANDERSSON M R, JANSSEN R A J, WANG Er-gang. High performance all-polymer solar cells by synergistic effects of fine-tuned crystallinity and solvent annealing [J]. Journal of the American Chemical Society, 2016, 138(34): 10935-10944. DOI: 10.1021/jacs.6b04822.
[10] ZHOU Liu-yang, HE Xuan, LAU T K, QIU Bei-bei, WANG Tao, LU Xin-hui, LUSZCZYNSKA B, ULANSKI J, XU Shu-tao, CHEN Guo-hui, YUAN Jun, ZHANG Zhi-guo, LI Yong-fang, ZOU Ying-ping. Nonhalogenated solvent-processed all-polymer solar cells over 7.4% efficiency from quinoxaline-based polymers [J]. ACS Applied Materials & Interfaces, 2018, 10(48): 41318-41325. DOI: 10.1021/acsami. 8b13949.
[11] YANG Jing, XIAO Bo, TANG Ai-ling, LI Jian-feng, WANG Xiao-chen, ZHOU Er-jun. Aromatic-diimide-based n-type conjugated polymers for all-polymer solar cell applications [J]. Advanced Materials, 2019, 31(45): 1804699. DOI: 10.1002/adma.201804699.
[12] WU Jing-nan, MENG Yuan, GUO Xia, ZHU Lei, LIU Feng, ZHANG Mao-jie. All-polymer solar cells based on a novel narrow-bandgap polymer acceptor with power conversion efficiency over 10% [J]. Journal of Materials Chemistry A, 2019, 7(27): 16190-16196. DOI: 10.1039/c9ta04611a.
[13] YAO Hua-tong, BAI Fu-jin, HU Hua-wei, ARUNAGIRI L, ZHANG Jian-quan, CHEN Yu-zhong, YU Han, CHEN Shang-shang, LIU Tao, LAI J Y L, ZOU Ying-ping, ADE H, YAN He. Efficient all-polymer solar cells based on a new polymer acceptor achieving 10.3% power conversion efficiency [J]. ACS Energy Letters, 2019, 4(2): 417-422. DOI: 10.1021/acsenergylett.8b02114.
[14] FAN Qun-ping, AN Qiao-shi, LIN Yuan-bao, XIA Yu-xin, LI Qian, ZHANG Ming, SU Wen-yan, PENG Wen-hong, ZHANG Chun-feng, LIU Feng, HOU Lin-tao, ZHU Wei-guo, YU Dong-hong, XIAO Min, MOONS E, ZHANG Fu-jun, ANTHOPOULOS T D, INGANAS O, WANG Er-gang. Over 14% efficiency all-polymer solar cells enabled by a low bandgap polymer acceptor with low energy loss and efficient charge separation [J]. Energy & Environmental Science, 2020, 13(12): 5017-5027. DOI: 10.1039/D0EE01828G.
[15] SUN Hui-liang, YU Han, SHI Yong-qiang, YU Jian-wei, PENG Zhong-xiang, ZHANG Xian-he, LIU Bin, WANG Jun-wei, SINGH R, LEE J, LI Yong-chun, WEI Zi-xiang, LIAO Qiao-gan, KAN Zhi-peng, YE Long, YAN He, GAO Feng, GUO Xu-gang. A narrow-bandgap n-type polymer with an acceptor-acceptor backbone enabling efficient all-polymer solar cells [J]. Advanced Materials, 2020, 32(43): 2004183. DOI: 10.1002/adma.202004183.
[16] YUAN Jun, ZHANG Yun-qiang, ZHOU Liu-yang, ZHANG Gui-chuan, YIP H L, LAU T K, LU Xin-hui, ZHU Can, PENG Hong-jian, JOHNSON P A, LECLERC M, CAO Yong, ULANSKI J, LI Yong-fang, ZOU Ying-ping. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core [J]. Joule, 2019, 3(4): 1140-1151. DOI: 10.1016/j.joule.2019.01.004.
[17] FENG Liu-liu, YUAN Jun, ZHANG Zhen-zhen, PENG Hong-jian, ZHANG Zhi-Guo, XU Shu-tao, LIU Ye, LI Yong-fang, ZOU Ying-ping. Thieno[3,2-b]pyrrolo-fused pentacyclic benzotriazole-based acceptor for efficient organic photovoltaics [J]. ACS Applied Materials & Interfaces, 2017, 9(37): 31985-31992. DOI: 10.1021/acsami.7b10995.
[18] LIU Sha, YUAN Jun, DENG Wan-yuan, LUO Mei, XIE Yuan, LIANG Quan-bin, ZOU Ying-ping, HE Zhi-cai, WU Hong-bin, CAO Yong. High-efficiency organic solar cells with low non-radiative recombination loss and low energetic disorder [J]. Nature Photonics, 2020, 14(5): 300-305. DOI: 10.1038/s41566-019-0573-5.
[19] YUAN Jun, ZHANG Yun-qiang, ZHOU Liu-yang, ZHANG Chu-jun, LAU T K, ZHANG Gui-chuan, LU Xin-hui, YIP H L, SO S K, BEAUPRE S, MAINVILLE M, JOHNSON P A, LECLERC M, CHEN Hong-gang, PENG Hong-jian, LI Yong-fang, ZOU Ying-ping. Fused Benzothiadiazole: A building block for n-type organic acceptor to achieve high-performance organic solar cells [J]. Advanced Materials, 2019, 31(17): 1807577. DOI: 10.1002/adma.201807577.
[20] JIANG Kui, WEI Qing-ya, LAI J Y L, PENG Zheng-xing, KIM H K, YUAN Jun, YE Long, ADE H, ZOU Ying-ping, YAN He. Alkyl chain tuning of small molecule acceptors for efficient organic solar cells [J]. Joule, 2019, 3(12): 3020- 3033. DOI: 10.1016/j.joule.2019.09.010.
[21] WEI Qing-ya, LIUWei, LECLERC M, YUAN Jun, CHEN Hong-gang, ZOU Ying-ping. A-DA′D-A non-fullerene acceptors for high-performance organic solar cells [J]. Science China Chemistry, 2020, 63(10): 1352-1366. DOI: 10.1007/ s11426-020-9799-4.
[22] CAO Rui, CHEN Yu, CAI Fang-fang, CHEN Hong-gang, LIU Wei, GUAN Hui-lan, WEI Qing-ya, LI Jing, CHANG Qin, LI Zhe, ZOU Ying-ping. A new chlorinated non-fullerene acceptor based organic photovoltaic cells over 12% efficiency [J]. Journal of Central South University, 2020, 27(12): 3581-3593. DOI: 10.1007/s11771-020-4501-0.
[23] JIA Tao, ZHANG Jia-bin, ZHONG Wen-kai, LIANG Yuan-ying, ZHANG Kai, DONG Sheng, YING Lei, LIU Feng, WANG Xiao-hui, HUANG Fei, CAO Yong. 14.4% efficiency all-polymer solar cell with broad absorption and low energy loss enabled by a novel polymer acceptor [J]. Nano Energy, 2020, 72: 104718. DOI: 10.1016/j.nanoen.2020.104718.
[24] LUO Mei, ZHOU Liu-yang, YUAN Jun, ZHU Can, CAI Fang-fang, HAI Jie-feng, ZOU Ying-ping. A new non-fullerene acceptor based on the heptacyclic benzotriazole unit for efficient organic solar cells [J]. Journal of Energy Chemistry, 2020, 42: 169-173. DOI: 10.1016/j.jechem. 2019.07.002.
[25] YUAN Jun, ZHANG Huo-tian, ZHANG Rui, WANG Yu-ming, HOU Jian-hui, LECLERC M, ZHAN Xiao-wei, HUANG Fei, GAO Feng, ZOU Ying-ping. Reducing voltage losses in the A-DA'D-A acceptor-based organic solar cells [J]. Chem, 2020, 6(9): 2147-2161. DOI: 10.1016/j.chempr.2020. 08.003.
[26] ZHOU Hua-xing, YANG Li-qiang, YOU Wei. Rational design of high performance conjugated polymers for organic solar cells [J]. Macromolecules, 2012, 45(2): 607-632. DOI: 10.1021/ma201648t.
[27] LU Lu-yao, ZHENG Tian-yue, WU Qing-he, SCHNEIDER A M, ZHAO Dong-lin, YU Lu-ping. Recent advances in bulk heterojunction polymer solar cells [J]. Chemical Reviews, 2015, 115(23): 12666-12731. DOI: 10.1021/acs.chemrev. 5b00098.
[28] YUAN Jun, HUANG Tian-yi, CHENG Pei, ZOU Ying-ping, ZHANG Huo-tian, YANG J L, CHANG Sheng-Yung, ZHANG Zhen-zhen, HUANG Wen-chao, WANG Rui, MENG Dong, GAO Feng, YANG Yang. Enabling low voltage losses and high photocurrent in fullerene-free organic photovoltaics [J]. Nat Commun, 2019, 10: 570. DOI: 10.1038/s41467-019-08386-9.
[29] PROCTOR C M, KIM C, NEHER D, NGUYEN T Q. Nongeminate recombination and charge transport limitations in diketopyrrolopyrrole-based solution-processed small molecule solar cells [J]. Advanced Functional Materials, 2013, 23(28): 3584-3594. DOI: 10.1002/adfm.201202643.
[30] CHEN Xiao-bin, XU Gui-ying, ZENG Guang, GU Hong-wei, CHEN Hai-yang, XU Hai-tao, YAO Hui-feng, LI Yao-wen, HOU Jian-hui, LI Yong-fang. Realizing ultrahigh mechanical flexibility and >15% efficiency of flexible organic solar cells via a “welding” flexible transparent electrode [J]. Advanced Materials, 2020, 32(14): 1908478. DOI: 10.1002/adma. 201908478.
[31] MIHAILETCHI V D, WILDEMAN J, BLOM P W. Space-charge limited photocurrent [J]. Physical Review Letters, 2005, 94(12): 126602. DOI: 10.1103/PhysRevLett.94. 126602.
[32] BLOM P W M, JONG M J M D, MUNSTER M G V. Electric-field and temperature dependence of the hole mobility in poly (p-phenylene vinylene) [J]. Physical Review B, 1997, 55: 656-659. DOI: doi.org/10.1103/PhysRevB.55.R656.
[33] MALLIARAS G G, SALEM J R, BROCK P J, SCOTT C. Electrical characteristics and efficiency of single-layer organic light-emitting diodes [J]. Physical Review B, 1998, 58: 13411-13414. DOI: /10.1103/PhysRevB.58. R13411.
(Edited by FANG Jing-hua)
中文导读
苯并三氮唑基窄带隙聚合物受体用于高效全聚合物太阳电池
摘要:合理设计具有强和宽吸收的聚合物受体有助于提高光伏性能。本文以七环苯并三氮唑(Y9-C16)为构筑单元,噻吩为连接单元合成一个新型聚合物受体材料PY9-T。该受体材料的薄膜具有较低的带隙(1.37 eV)和较高的消光系数(1.44×105 cm-1)。当PY9-T与宽带隙聚合物给体PBDB-T共混时,制备全聚合物太阳电池(APSC)实现10.45%的高能量转换效率,器件具有0.881 V的高开路电压和19.82 mA/cm2的高短路电流密度。此外,器件在100°C退火后,共混膜形貌仅发生微小的变化,表明APSC具有好的热稳定性,Y9-C16为开发高效稳定的聚合物受体提供了新的构筑单元。
关键词:全聚合物太阳电池;聚合物受体;窄带隙;苯并三氮唑;能量转换效率
Foundation item: Project(21875286) supported by the National Natural Science Foundation of China
Received date: 2021-01-09; Accepted date: 2021-04-28
Corresponding author: ZOU Ying-ping, PhD, Professor; Tel: +86-18711140102; E-mail: yingpingzou@csu.edu.cn; ORCID: https://orcid. org/0000-0003-1901-7243

