Adsorption kinetics and thermodynamics of sodium butyl xanthate onto bornite in flotation
来源期刊:中南大学学报(英文版)2019年第11期
论文作者:印万忠 孙乾予 曹少航 YANG Bin(杨斌) 孙浩然 唐远 王东辉 姚金
文章页码:2998 - 3007
Key words:bornite; sodium butyl xanthate; adsorption; kinetics and thermodynamics; infrared spectrum
Abstract: In this paper, the effect of sodium butyl xanthate (NaBX) adsorption on the surface of bornite at different pH on flotation was studied by adsorption kinetic and thermodynamic. The flotation results demonstrated that the recovery was the highest when pH was 9 in NaBX solution (4×10-5 mol/L). The adsorption kinetics showed the reaction of NaBX on the bornite conformed to the second order kinetic equation; it belonged to the multimolecular layer adsorption of Freundlich model; the maximum adsorption rate constant was 0.30 g/(10-6 mol·min), and the equilibrium adsorption capacity was 2.70×10-6 mol/g. Thermodynamic calculation results indicated that the adsorption process was spontaneous chemisorption, and the adsorption products of NaBX on bornite surface were cupric butyl xanthate, ferric butyl xanthate and dixanthogen, which were confirmed by infrared spectrum measurements.
Cite this article as: SUN Qian-yu, YIN Wan-zhong, CAO Shao-hang, YANG Bin, SUN Hao-ran, TANG Yuan, WANG Dong-hui, YAO Jin. Adsorption kinetics and thermodynamics of sodium butyl xanthate onto bornite in flotation [J]. Journal of Central South University, 2019, 26(11): 2998-3007. DOI: https://doi.org/10.1007/s11771-019-4231-3.

J. Cent. South Univ. (2019) 26: 2998-3007
DOI: https://doi.org/10.1007/s11771-019-4231-3

SUN Qian-yu(孙乾予)1, 2, YIN Wan-zhong(印万忠)2, 3, CAO Shao-hang(曹少航)2, YANG Bin(杨斌)2,
SUN Hao-ran(孙浩然)2, TANG Yuan(唐远)2, WANG Dong-hui(王东辉)2, YAO Jin(姚金)2
1. School of Environment, Tsinghua University, Beijing 100084, China;
2. School of Resources and Civil Engineering, Northeastern University, Shenyang 110819, China;
3. School of Zijin Mining, Fuzhou University, Fuzhou 350116, China
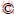 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: In this paper, the effect of sodium butyl xanthate (NaBX) adsorption on the surface of bornite at different pH on flotation was studied by adsorption kinetic and thermodynamic. The flotation results demonstrated that the recovery was the highest when pH was 9 in NaBX solution (4×10-5 mol/L). The adsorption kinetics showed the reaction of NaBX on the bornite conformed to the second order kinetic equation; it belonged to the multimolecular layer adsorption of Freundlich model; the maximum adsorption rate constant was 0.30 g/(10-6 mol·min), and the equilibrium adsorption capacity was 2.70×10-6 mol/g. Thermodynamic calculation results indicated that the adsorption process was spontaneous chemisorption, and the adsorption products of NaBX on bornite surface were cupric butyl xanthate, ferric butyl xanthate and dixanthogen, which were confirmed by infrared spectrum measurements.
Key words: bornite; sodium butyl xanthate; adsorption; kinetics and thermodynamics; infrared spectrum
Cite this article as: SUN Qian-yu, YIN Wan-zhong, CAO Shao-hang, YANG Bin, SUN Hao-ran, TANG Yuan, WANG Dong-hui, YAO Jin. Adsorption kinetics and thermodynamics of sodium butyl xanthate onto bornite in flotation [J]. Journal of Central South University, 2019, 26(11): 2998-3007. DOI: https://doi.org/10.1007/s11771-019-4231-3.
1 Introduction
As copper is featured by some physical and chemical properties, it has been widely applied in the fields of electrification, mechanical manufacturing, transportation, and construction [1, 2]. Metallic copper is generally obtained from the copper ore through a series of purification processes, including ore mining, mineral processing and smelting [3]. Specifically, two main methods are dominant in the purification of copper ore, namely flotation and leaching, which are applicable for sulfide copper ore and oxidized copper ore, respectively [4]. Most copper (about 80%) in the world is obtained from sulfide copper ore (mainly involving chalcopyrite, chalcocite, and bornite) through flotation. Based on different physicochemical properties of mineral particle surfaces, the flotation process can be realized by adding agents with selective adsorption properties [3, 5-7].
As the collector, xanthate is the most widely used one in the flotation of copper ore [7-9]. The formula of xanthate is R-OCSSM, where R is the alkyl group with different chain lengths (C1-8), and M is the metal ion (K+ or Na+). Functional group of OCS2 has a strong adsorption capacity for copper ions [10]. It is necessary to investigate the adsorption thermodynamics and kinetics of xanthate on copper sulfide for improved separation efficiency. According to Mustafa’s study based on the adsorption kinetics theory, the adsorption of xanthate ion on chalcopyrite surface conforms to Lagrergren’s first-order rate equation [11]. NAEEM et al [12] found that the adsorption between xanthate ion and chalcocite surface was spontaneous, and they calculated the adsorption active energy. According to numerous industrial practices, chalcocite is demonstrated to have stronger floatability than chalcopyrite in neutral or alkalescent pH range [13-15], which is probably caused by the different hydrophobic products on each mineral surface [16-18]. MORENO- MEDRANO et al [19] identified the xanthate products on the surface of chalcopyrite as covellite and cuprous xanthate through voltammetry and electrochemical impedance spectroscopy (EIS). Leppinnen indicated the xanthate products on chalcopyrite surface under alkalescent conditions (pH=9.2) to be copper xanthate and dixanthogen through in-situ FT-IR, while the products on the surface of chalcocite to be copper xanthate with polymolecular layer [20]. According to the work by MENDIRATTA [21] adopting Tafel curve changes, the xanthate products on the surfaces of chalcocite and covellite are cuprous xanthate. Current studies about xanthate adsorption and corresponding products mainly highlight chalcopyrite and chalcocite, with insufficient attention paid to adsorption behavior and mechanism between xanthate and bornite. Bornite commonly exists in copper deposits with high abundance, and its existence can influence the flotation results of sulfide copper ores. Therefore, it should be comprehensively investigated in order to improve the floatation effects.
In this study, the flotation behaviors of bornite using NaBX as a collector were investigated through flotation tests; the adsorption mechanism of NaBX on bornite surface was analyzed by calculation of adsorption kinetics and thermodynamics; the adsorption products were identified by the Fourier transform infrared (FTIR) spectroscopy.
2 Experimental
2.1 Materials and reagents
Bornite samples were provided by Dexing Copper Mine in Jiangxi Province, China, and milled by ceramic ball mill after crushing and manual picking. The particle with sizes of 37-74 μm were used for flotation and adsorption experiments, while the particles with size ≤37 μm were milled by agate mortar for X-ray diffraction analyses (see Figure 1) and chemical analysis (see Table 1). Figure 1 shows that the bornite sample was pure without other obvious miscellaneous peaks, and Table 1 illustrats that the 61.07% of Cu was contained in the bornite sample, and the purity calculated with Cu as the benchmark was 96.48%.
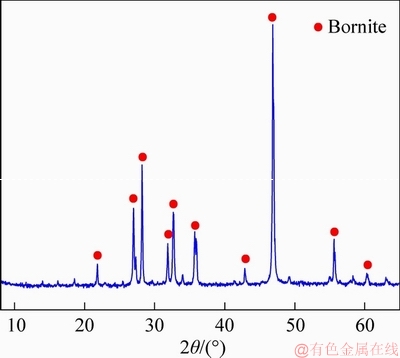
Figure 1 X-ray diffraction pattern of bornite
Table 1 Composition of bornite sample (wt %)

Chemical grade sodium butyl xanthate (NaBX) was provided by Tieling Pharmaceutical Co., Ltd in Liaoning Province, China. Cupric butyl xanthate (Cu(BX)2) was made by mixing the same volume of sodium butyl xanthate solution (1×10-3 mol/L) and cupric sulfate solution (1×10-3 mol/L), associated with strong stirring, filtering, cleaning with hexane, and vacuum drying at 313 K. According to previous research, the dixanthogen on the cupric butyl xanthate can be cleaned out with hexane in the preparation [22]. Ferric butyl xanthate (Fe(BX)3) was made using the same method for preparation of cupric butyl xanthate. Dibutyl dixanthogen ((BX)2) was prepared in 1×10-3 mol/L sodium butyl xanthate solution, with gradual addition of the same volumetric concentration of KI3 solution. Deionized water was used in preparation of solutions for all experiments.
2.2 Methods
2.2.1 Micro-flotation tests
Micro-flotation tests were carried out in a mechanical flotation cell (mode: XFG; volume:40 mL) at a constant agitation rate of 1600 r/min. In each test, 2 g of pure minerals and 35 mL of deionized water were placed into the tank, with hydrochloric acid or sodium hydroxide used for pH adjustment. After 3 min stirring by pulp, a desired amount of NaBX was added and conditioned for another 3 min. Then frother MIBC (methyl isobutyl carbinol) was put in and stirred for 1 min, with manual flotation for 3 min. Finally, the froth concentrate and tailings inside the tank were respectively dried and weighed to calculate the recovery.
2.2.2 Adsorption experiments
In each adsorption test, 0.5 g of pure bornite was placed with 100 mL of aqueous sodium butyl xanthate solution in a flask, and the pH value was adjusted with NaOH or HCl solution. Afterwards, the liquid supernatant was shaken in a temperature-controlled water bath at 293 K for 10 min. The suspensions were filtered through centrifugation, and the concentration of residual xanthate was measured by UV-visible spectrophotometer with wave length of 301 nm. According to the initial concentration of xanthate, the adsorbing capacity of xanthate on the mineral surface was calculated as
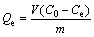 (1)
(1)
where Qe is the adsorbing capacity; V is the solution volume; C0 is the initial concentration of xanthate; Ce is the concentration of residual xanthate; m is the mineral mass.
2.2.3 FT-IR spectra analysis
The amounts of sodium butyl xanthate, metal xanthate salt and bornite before and after agent addition were measured using Nicolet 380 FT-IR spectrometer in the range of 400-4000 cm-1. The oily dixanthogen liquid dropping on the pelleted purified KBr was measured, where the purified KBr was adopted as the collecting background.
3 Results and discussion
3.1 Micro-flotation test
Flotation performance of bornite at different NaBX concentrations and pH values is shown in Figure 2. It can be seen that the recovery of bornite increased with the increase of NaBX concentration, reaching over 80% when the concentration reached 4×10-5 mol/L in the conditions with pH values of 5, 7 and 9. Moreover, it could be as high as 90% with the pH value of 9. It is possible that more NaBX can be adsorbed on the bornite surface. However, with the pH value of 12, the recovery rised slowly with NaBX concentration increase, only reaching 60% as the concentration is 8×10-5 mol/L. Therefore, it can be concluded that NaBX has a good flotation effect on bornite in the pH rang from weak acidic to weak alkaline, while strong alkaline condition (pH 12) is not.
Figure 2 Recovery of bornite with different NaBX concentrations at different pH values
3.2 Adsorption kinetics
At room temperature (293 K), the adsorption experiments were performed at different pH values in the NaBX solution (4×10-5 mol/L). The adsorption curve is shown in Figure 3. It can be seen that as pH increases from 5 to 9 and the adsorption time extends, the adsorption capacity increases sharply at first and then increases slowly, finally reaches the adsorption equilibrium, with the maximum adsorption capacity increased from 1.95×10-6 mol/g to 2.68×10-6 mol/g. Under strong alkaline condition (pH=12), the adsorption capacity increases slowly, with its maximum of 1.32×10-6 mol/g. At different pH conditions, the adsorption equilibrium time is 12 min proximately.

Figure 3 Adsorption capacity of NaBX in bornite with variety of pH values at different times
Pseudo-first and second order kinetics adsorption models were used to describe the adsorption process and the expression of pseudo-first order kinetics adsorption model is as follows:
 (2)
(2)
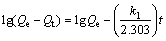 (3)
(3)
where Qe represents the adsorption capacity of xanthate on bornite in adsorption equilibrium, mol/g; Qt is the adsorption capacity of xanthate on bornite at time t, mol/g; k1 is the adsorption rate constant in the pseudo-first order kinetics, min-1; the experimental data are carried out by linear fitting with t as abscissa, and lg(Qe-Qt) as ordinate, as shown in Figure 4.
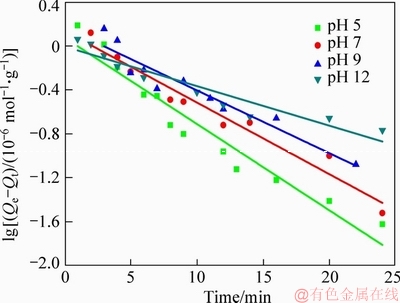
Figure 4 Linear fit of pseudo-first order kinetics
The expression of the pseudo-second order kinetics adsorption model is as follows:
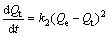 (4)
(4)
By integral transformation, the above equation can be written as:
 (5)
(5)
where k2 is the adsorption rate constant in pseudo- second order kinetics, g/(mol·min); the experimental data are carried out by linear fitting with t as abscissa, and t/Qt as ordinate, as shown in Figure 5.
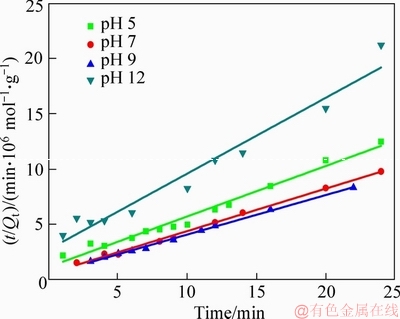
Figure 5 Linear fitting of pseudo-second order kinetics
Table 2 indicates that under different pH conditions, the R2 value of first-order kinetics rate equation was in the range of 0.89 to 0.95, with low correlation coefficient, and the calculated value Qe(cal) was far below the experimental value Qe(exp), which demonstrates that the above equation violated the first-order kinetics rate equation. The linear determined coefficient R2 of pseudo-second order kinetics rate equation was higher, being 0.98, 0.99, 0.99 and 0.96, respectively. The calculated adsorption equilibrium value Qe(cal) was closer to the experimental value Qe(exp), indicating that the adsorption process of NaBX on bornite surface can be better described by the pseudo-second order kinetics model. Chemical bonding is the main factor affecting pseudo-second order kinetics adsorption, and hence it can be inferred that chemisorption is the main reaction occurring on bornite surface [23].
3.3 Adsorption isotherm test and equation fitting
During the process of micro-flotation test, Langmuir and Freundlich equations were adopted to describe the adsorption model of agent acting on mineral surface [24-26]. At the room temperature (293 K), initial concentrations of NaBX were 1× 10-5, 2×10-5, 4×10-5, 8×10-5 and 10×10-5 mol/L, respectively, and the adsorption isotherms of NaBX on bornite were measured under different pH conditions, as illustrated in Figure 6. The adsorption process was described with Langmuir and Freundlich isotherm equations to fit data in Figure 6. The linear expression of Langmuir equation is as follows:
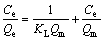 (6)
(6)
The linear expression of Freundlich equation is as below:
 (7)
(7)
where Ce is the concentration of residual xanthate in the solution during adsorption equilibrium, mol/g; Qe is the adsorption capacity of xanthate on bornite during adsorption equilibrium, mol/g; Qm is the maximum monolayer adsorption capacity, mol/g; KL and KF are adsorption constants related to the maximum adsorption capacity; and 1/n is the unevenness of adsorption. The fitting results obtained by linear regression equation are shown in Table 3. KF value in Freundlich equation rises from 0.61 to 1.01, and all n values are greater than 1, when pH ranges from 5 to 9. It shows that the adsorption process is easy to proceed, and bornite has a high adsorption for xanthate, with a larger R2 correlation coefficient. It can be suggested that the adsorption of xanthate on bornite in the Freundlich equation can be better described as multilayer adsorption, while under strong alkaline condition (pH=12) RL2>RF2, the adsorption data of xanthate on bornite are more aligned with Langmuir adsorption isotherm model, which indicates that the adsorption might be monolayer adsorption in this case.
Table 2 Adsorption kinetic parameters of NaBX on bornite surfaces


Figure 6 Adsorption isotherm under different pH
3.4 Adsorption thermodynamics calculation
The adsorption of NaBX on bornite can induce thermodynamic changes of the system. At 293 K, 303 K and 313 K, the equilibrium adsorption capacity and equilibrium concentration are shown in Figure 7. Thermodynamic parameters of NaBX acting on bornite are calculated according to Van’t Hoff Eqs. (8) and (9):
 (8)
(8)
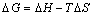 (9)
(9)
The following equation is obtained based on simultaneous equations of Eqs. (8) and (9):
 (10)
(10)
where △H is the enthalpy change, kJ/mol; △S is the entropy change, J/(mol·K); R , as the perfect gas constant, is assigned to be 8.314 J/(mol·K); and T is the thermodynamic temperature, K. The lnK is the linear intercept obtained by the relationship between ln(Qe/Ce) and Qe as well as the earlier work by KHAN et al [27], as shown in Figure 8. Through plotting and fitting of lnK-T -1 (Figure 9), two straight lines with favorable linear relationships were obtained. The slopes (-△H/R) were 4490.2 and 3767.2, respectively, while the intercepts (△S/R) were -14.08 and -11.84, respectively. Moreover, the thermodynamic parameters can be calculated according to Eqs. (8) and (9), which are shown in Table 4.
Through the analysis of data in Table 4, △G<0, indicating the adsorption was a spontaneous process; △H<0, indicating that the adsorption of NaBX on bornite was an exothermal process; under the same conditions, △GpH=9<△GpH=12, suggesting that the adsorption reaction was more likely to occur at pH 9. According to the previous research, physical adsorption occurs in the case that the absolute value △H is within the range of 2.1 to 20.9 kJ/mol [28], while chemisorption is infered when the value is within the range of 20.9 to 418.4 kJ/mol. The △H calculated in this paper was -37.22 and -30.89 kJ/mol, respectively, based on which conclusions can be arrived that the adsorption of NaBX on bornite might be chemisorption.
Table 3 Langmuir and Freundlich adsorption isotherm equation and parameters
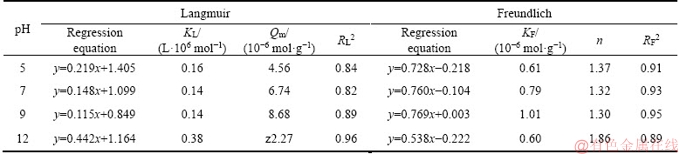

Figure 7 Adsorption isotherm of NaBX on bornite at pH 9 (a) and pH 12 (b)

Figure 8 Fitting straight-line of ln(Qe/Ce)-Qe at various temperatures and pH 9 (a) and pH 12 (b)

Figure 9 Fitting curve of lnK-T -1
Table 4 Thermodynamics calculation results of butyl xanthate adsorbed on bornite

3.5 Infrared spectra of NaBX, (BX)2, Cu(BX)2 and Fe(BX)3
Figure 10(a) represents the infrared spectrum of NaBX, in which 1062.6 cm-1 was the stretching vibration peak of bond C=S; 1110.8 cm-1 was the symmetric stretching vibration peak of bond C—O—C; 1152.3 and 1172.8 cm-1 were the asymmetric stretching vibration peaks of bond C—O—C. Figure 10(b) represents the infrared spectrum of (BX)2, in which 1042.6 cm-1 was the stretching vibration peak of bond C=S; 1217.3 and 1263.2 cm-1 were the asymmetric stretching vibration peaks of bond C—O—C; 1120.4 cm-1 was the symmetric stretching vibration peak of bond C—O—C. Figure 10(c) illustrates the infrared spectrum of Cu(BX)2, in which 1025.3 cm-1 was the stretching vibration peak of bond C=S; 1125.4 and 1173.4 cm-1 were respectively the stretching vibration peaks of bond C—O—C; 1204.2 cm-1 was the asymmetric stretching vibration peak of bond C—O—C. Figure 10(d) presents the infrared spectrum of Fe(BX)3, in which 1025.1 cm-1 was the stretching vibration peak of bond C=S; 1217.3 and 1263.2 cm-1 were respectively the asymmetric stretching vibration peaks of bond C—O—C; and 1140.2 cm-1 was the symmetric stretching vibration peak of bond C—O—C [29, 30]. The main characteristic vibration adsorption peaks of NaBX, (BX)2, Cu(BX)2 and Fe(BX)3 listed in Table 5, were used as the basis for determining adsorption products of NaBX on bornite.
3.6 FTIR spectral frequencies of NaBX adsorbed on bornite
Figure 11 demonstrates the infrared spectra under different pH conditions before and after the reaction of bornite with NaBX. Figure 11(a) shows the infrared spectrum of bornite, in which a strong characteristic peak appeared at 1400 cm-1, and it was supposed to be the in-plane bending vibration peak of bond —OH on the hydroxylated surface of bornite, which may be caused by bornite oxide in water. Figure 11(b) presents the infrared spectrum after bornite reacting with NaBX at pH 5. According to Figures 10(a)-(c), it can be found through comparison of infrared spectra of (BX)2, Cu(BX)2 and Fe(BX)3 that the characteristic peaks at 1023.5, 1125.1 and 1201.4 cm-1 were consistent with the stretching vibration peak of bond C=S and the symmetrical and asymmetric stretching vibration peaks of bond C—O—C in Cu(BX)2; the characteristic peaks at 1042.8 and 1265.1 cm-1 correspond to the stretching vibration peak of bond C=S and the asymmetric stretching vibration peak of bond C—O—C in (BX)2, respectively; the characteristic peak at 1140.1 cm-1 was basically identical to the symmetric stretching vibration peak of bond C—O—C in Fe(BX)3, suggesting that (BX)2 may bind to the metal butyl xanthate salts on mineral surface by hydrogen bond [31]. Figure 11(c) represents the infrared spectra after bornite reacting with NaBX at pH 9, in which the characteristic peaks at 1023.6, 1041.7, 1125.1, 1140.0, 1201.9 and 1266.2 cm-1 were consistent with those in Figure 11(b), indicating that the products on mineral surface in the condition with pH 9 was basically the same with those with pH 5. It indicates that the adsorption products of NaBX on the bornite were (BX)2, Cu(BX)2 and Fe(BX)3 under weak acid to weak alkali condition. Figure 11(d) presents the infrared spectra after bornite reacting with butyl NaBX at pH 12; the characteristic peaks at 1122.4 and 1166.5 cm-1 were consistent with the symmetric stretching vibration peak of bond C—O—C in Cu(BX)2, and they shift to the direction of low frequency. The characteristic peak at 1025.4 cm-1 corresponds to the stretching vibration peak of bond C=S in Cu(BX)2; the characteristic peak at 1206.3 cm-1 corresponds to the asymmetric stretching vibration peak of bond C—O—C in Cu(BX)2, and no characteristic peak can be found in (BX)2 and Fe(BX)3. It indicated that the adsorbed product of NaBX on bornite was only Cu(BX)2 under strong alkali condition.
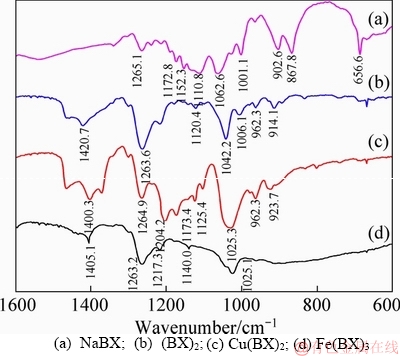
Figure 10 Infrared spectra of:
Table 5 FTIR spectral frequencies of xanthate and metal xanthate
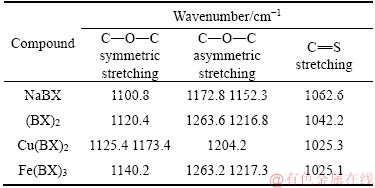

Figure 11 Infrared spectra of NaBX adsorbed on bornite surface:
4 Conclusions
1) Micro-flotation test shows that NaBX had good floatability for bornite at pH 5-9, but it had poor flotability at strong alkali condition (pH=12).
2) The adsorption measurement and kinetic calculation suggest that the adsorption of NaBX on bornite surface conformed to the second-order kinetic equation, the maximum adsorption rate constant was 0.30 g/(10-6 mol·min), and the equilibrium adsorption capacity was 2.68×10-6 mol/g. When the pH value was within the range from 5 to 9, the adsorption of butyl xanthate conforms to the multimolecular layer adsorption of Freundlich Mode; when the pH value was 12, the adsorption conformed to monolayer adsorption of Langmuir model.
3) The infrared spectroscopic analysis and thermodynamic calculation indicate that the reaction of NaBX on bornite surface was a spontaneously proceeding chemisorption process; the adsorbed species contain Cu(BX)2, Fe(BX)3 and (BX)2 under weak acid to weak alkali condition, while there was only Cu(BX)2 under strong alkali condition.
References
[1] LANZ B, RUTHERFORD T F, TILTON J E. Subglobal climate agreements and energy-intensive activities: An evaluation of carbon leakage in the copper industry [J]. World Economy, 2013, 36(3): 254-279.
[2] ROBERTS M C. Metal use and the world economy [J]. Resources Policy, 1996, 22(3): 183-196.
[3] ROY S, DATTA A, REHANI S. Flotation of copper sulphide from copper smelter slag using multiple collectors and their mixtures [J]. International Journal of Mineral Processing, 2015, 143: 43-49.
[4] LI Fang-xu, ZHONG Hu, XU Hai-feng. Flotation behavior and adsorption mechanism of α-hydroxyoctyl phosphinic acid to malachite [J]. Minerals Engineering, 2015, 71: 188-193.
[5] LUO Xi-mei, WANG Yun-fan, WEN Shu-ming, MA Ming-ze, SUN Chao-yao, YIN Wan-zhong, MA Ying-qiang. Effect of carbonate minerals on quartz flotation behavior under conditions of reverse anionic flotation of iron ores [J]. International Journal of Mineral Processing, 2016, 152: 1-6.
[6] LUO Xi-mei, YIN Wan-zhong, WANG Yun-fan, SUN Chuan-yao, MA Ying-qiang, LIU Jian. Effect and mechanism of siderite on reverse anionic flotation of quartz from hematite [J]. Journal of Central South University, 2016, 23(1): 52-58.
[7] LEE K, ARCHIBALD D, MCLEAN J, REUTER M A. Flotation of mixed copper oxide and sulphide minerals with xanthate and hydroxamate collectors [J]. Minerals Engineering, 2009, 22(4): 395-401.
[8] VREUGDENHIL A J, FINCH J A, BUTLER I S. Analysis of alkylxanthate collectors on sulphide minerals and flotation products by headspace analysis gas-phase infrared spectroscopy (HAGIS) [J]. Minerals Engineering, 1999, 12(7): 745-756.
[9] KOWAL A, POMIANOWSKI A. Cyclic voltammetry of ethyl xanthate on a natural copper sulphide electrode [J]. Journal of Electroanalytical Chemistry, 1973, 46(2): 411-420.
[10] LOTTER N O, BRADSHAW D J. The formulation and use of mixed collectors in sulphide flotation [J]. Minerals Engineering, 2010, 23(11): 945-951.
[11] MUSTAFA S, HAMID A, NAEEM A. Xanthate adsorption studies on chalcopyrite ore [J]. International Journal of Mineral Processing, 2004, 74(1-4): 317-325.
[12] NAEEM A, ALI H, MUSTAFA S. Kinetics of xanthate sorption by copper sulphide (CuS) [J]. Journal-Chemical Society of Pakistan, 2008, 30(4): 517-520.
[13] CHEN Xu-meng, PENG Yong-jun, BRADSHAW D. The separation of chalcopyrite and chalcocite from pyrite in cleaner flotation after regrinding [J]. Minerals Engineering, 2014, 58(4): 64-72.
[14] HASEGAWA M. The practical application of shs for chalcopyrite flotation at Yaguki mill [J]. Resources Processing, 1969, 39: 21-25.
[15] OWUSU C, ABREU S, SKINNER W. The influence of pyrite content on the flotation of chalcopyrite/pyrite mixtures [J]. Minerals Engineering, 2014, 55(1): 87-95.
[16] MAIER G S, DOBI
 B. 2-mercaptobenzothiazole and derivatives in the flotation of galena, chalcocite and sphalerite: A study of flotation, adsorption and microcalorimetry [J]. Minerals Engineering, 1997, 10(12): 1375-1393.
B. 2-mercaptobenzothiazole and derivatives in the flotation of galena, chalcocite and sphalerite: A study of flotation, adsorption and microcalorimetry [J]. Minerals Engineering, 1997, 10(12): 1375-1393.
[17] QU Xiao-yan, XIAO Jing-jing, LIU Guang-yi, LI Sheng, ZHANG Zhi-yong. Investigation on the flotation behavior and adsorption mechanism of 3-hexyl-4-amino-1,2, 4-triazole-5-thione to chalcopyrite [J]. Minerals Engineering, 2016, 89: 10-17.
[18] HANGONE G, BRADSHAW D, EKMEKCI Z. Flotation of a copper sulphide ore from Okiep using thiol collectors and their mixtures [J]. Journal-South African Institute of Mining and Metallurgy, 2005, 105(3): 199-206.
[19] MORENO-MEDRANO E D, CASILLAS N, CRUZ R. Adsorption study of sodium isopropyl xanthate on chalcopyrite [J]. ECS Transactions, 2013, 47(1): 69-75.
[20] LEPPINEN J O, BASILIO C I, YOON R H. In-situ FTIR study of ethyl xanthate adsorption on sulfide minerals under conditions of controlled potential [J]. International Journal of Mineral Processing, 1989, 26(3, 4): 259-274.
[21] MENDIRATTA N K. Kinetic studies of sulfide mineral oxidation and xanthate adsorption [J]. Dissertation Abstracts International, 2000, 61(6): 3236-3398.
[22] MONTALTI M, FORNASIERO D, RALSTON J. Ultraviolet-visible spectroscopic study of the kinetics of adsorption of ethyl xanthate on pyrite [J]. Journal of Colloid & Interface Science, 1991, 143(91): 440-450.
[23] HO Y S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods [J]. Water Research, 2006, 40(1): 119-125.
[24] KWON K C, ROHRER R L, LAI R W. Floatabilities of treated coal in water at room temperature [J]. Separation Science & Technology, 1995, 30(7-9): 1997-2020.
[25] HELBIG C, BALDAUF H, MAHNKE J. Investigation of Langmuir monofilms and flotation experiments with anionic/cationic collector mixtures [J]. International Journal of Mineral Processing, 1998, 53(3): 135-144.
[26] NOVICH B E, RING TA. A predictive model for the alkylamine-quartz flotation system [J]. Langmuir, 1985, 1(6): 701-708.
[27] KHAN A A, SINGH R P. Adsorption thermodynamics of carbofuran on Sn (IV) arsenosilicate in H+, Na+ and Ca2+ forms [J]. Colloids & Surfaces, 1987, 24(1): 33-42.

