文章编号:1004-0609(2013)06-1764-05
高含量NaOH体系中Mg2SiO4的浸出机理
赵昌明,翟玉春
(东北大学 材料冶金学院,沈阳 110004)
摘 要:以Mg(OH)2·4MgCO3·6H2O和SiO2为原料,采用高温固相法合成Mg2SiO4,利用XRD和Raman光谱表征其结构。通过正交试验,优化高含量NaOH中Mg2SiO4的浸出反应条件,得出最佳试验条件为:温度220 ℃,时间120 min,液固比6:1,NaOH溶液含量85%(质量分数)。在优化试验基础上,采用拉曼光谱对碱浸出过程进行在线检测,利用X射线衍射仪分析碱浸后的水浸渣,研究高含量NaOH中Mg2SiO4浸出反应机理,结果表明:在反应过程中硅氧四面体中的Si—O键被破坏,NaOH介入硅酸盐晶格中,Mg2+经过碱浸过程可以脱离SiO4阵列,以Mg(OH)2形式从其硅酸盐中得以释放。
关键词:Mg2SiO4;NaOH溶液;拉曼光谱;反应机理
中图分类号:TF11.31 文献标志码:A
Leaching behavior mechanism of Mg2SiO4 in high NaOH content system
ZHAO Chang-ming, ZHAI Yu-chun
(School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China)
Abstract: Mg2SiO4 was finely prepared by high temperature solid state method using Mg(OH)2·4MgCO3·6H2O and SiO2 as raw materials. The structure of Mg2SiO4 was investigated by XRD and Raman spectroscopy. The optimized conditions for dissolution reaction of Mg2SiO4 in high NaOH content system were investigated by orthogonal test, and the optimal parameters were obtained as follows: reaction temperature of 220 ℃, reaction time of 120 min, liquid-solid ratio of 6:1 and NaOH content of 85% (mass fraction). Based on the optimal experiment, the leaching reaction mechanism of Mg2SiO4 in high NaOH content system was investigated. Raman spectra were measured for the reactions between the silicates and sodium hydroxide in-situ during the alkali dissolution process. Meanwhile, the water-leaching residue of the dissolution products was characterized by X-ray diffractometry. The results show that the bindings Si—O in silicates can be destroyed by aggression of sodium hydroxide, the magnesium ions in Mg2SiO4 can be separated from the silica arrays and liberated in the form of Mg(OH)2 from the silicates.
Key words: Mg2SiO4; NaOH solution; Raman spectrum; reaction mechanism
在当前全球经济快速发展和对金属镍需求持续上升的形势下,随着硫化镍矿资源和高品位红土镍矿资源的逐渐减少,大量品位在1%左右的红土镍矿的经济利用问题日益受到关注[1-4]。因此,如何高效率、低成本地处理低品位红土镍矿,以满足镍需求量的增加显得尤为重要。碱法处理硅酸盐形式的红土镍矿(Ni2SiO3·mMg2SiO4·nH2O),因其工艺简单、能耗低、投资少而日益受到人们的广泛关注。碱法处理红土镍矿的反应过程中,存在Mg2SiO4与NaOH的反应阶段,因此,有必要对硅酸盐的转化进行研究。
目前,国内外文献关于红土镍矿的生产工艺研究很多,而有关红土镍矿中的矿物与碱反应机理的研究报道较少。本文作者采用Raman光谱研究Mg2SiO4在高NaOH含量体系中的转化行为和转化机制,为硅酸盐形式的红土镍矿的合理利用提供参考。
1 实验
1.1 实验仪器
采用日本理学Rigaku X射线衍射仪分析样品物相;采用Jobin-Y'von公司的LabRAM HR-800型拉曼光谱仪测试样品结构,并配以脉冲激光光源和增强型电荷耦合探测器(Intensifie charge coupled device,ICCD),光源采用半导体脉冲激光器,所用激光波长为532 nm,激光平均功率为0.84 W,脉冲频率为25 kHz,狭缝宽度为500 μm,脉冲时间为10 ns,扫面波数范围为0~4 000 cm-1。
反应器由不锈钢制成,采用加热套加热,通过KWT型可控硅温度控制器控温,用镍铬-镍硅热电耦测温,温控精度为±2 ℃,反应釜上装有搅拌装置和回流冷凝管,其接口处、采样口以及釜盖均采取密封措施,实验装置如图1所示。
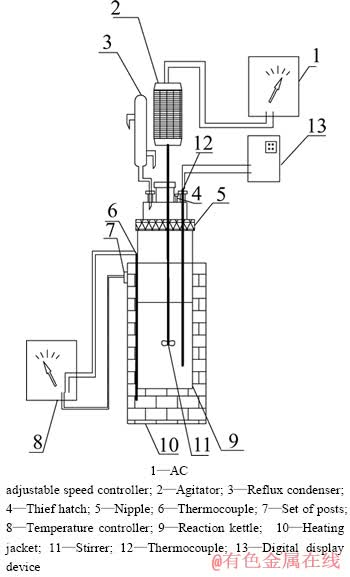
图1 实验装置示意图
Fig. 1 Schematic diagram of experimental apparatus
1.2 实验方法
1.2.1 Mg2SiO4的合成
根据Mg2SiO4的化学组成,称取一定质量的Mg(OH)2·4MgCO3·6H2O和SiO2,机械混料6h后放入炉内焙烧。
1.2.2 Mg2SiO4在高NaOH含量体系中的浸出
称取一定量NaOH放入反应釜中,加去离子水至设定含量,按一定的液固比(NaOH溶液质量与Mg2SiO4的质量比)加入Mg2SiO4,开通冷凝水,搅拌,加热到设定的温度。反应到达指定时间后,加水稀释,体系温度降至100℃时,开釜取料,过滤,通过离心分离得到含可溶性硅酸盐的浸出液和碱浸水浸渣,浸出液中二氧化硅含量采用氟化钠滴定法测定。
1.2.3 Mg2SiO4在高NaOH含量体系中Raman光谱的测定
称取一定量NaOH放入玛瑙研钵中,加入去离子水至设定浓度,按一定的液固比(NaOH溶液质量与Mg2SiO4的质量比)加入Mg2SiO4,在研钵中研细混合,以确保粉末充分混合均匀。用药品匙取样品少量放入直径为5 mm的铂坩埚中,然后将铂坩埚放入高温显微热台进行拉曼光谱测定。
2 结果与讨论
2.1 Mg2SiO4的结构表征
2.1.1 Mg2SiO4的物相分析
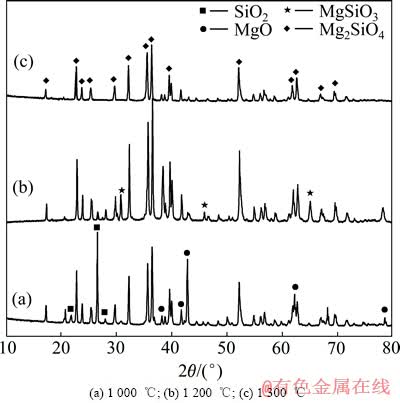
图2 不同温度熔烧后Mg2SiO4的XRD谱
Fig. 2 XRD patterns of Mg2SiO4 after sintered at different temperatures
图2所示为分别经1 000、1 200和1 300 ℃焙烧6 h后Mg2SiO4的XRD谱。由图2可知,Mg2SiO4的形成反应在1 000 ℃已基本完成,但是晶体长大和烧结都进行得很慢,体系中存在未反应的SiO2和MgO;在1 200 ℃时,体系中出现顽火辉石(MgSiO3)相,说明在此温度下SiO2和MgO反应生成MgSiO3;而在1 300 ℃时,体系中没有出现MgSiO3相[5],这是由于温度升高后,随反应进行MgSiO3与包裹在其表面的MgO反应生成Mg2SiO4所致,即
MgSiO3+MgO→Mg2SiO4 (1)
反应产物主要峰的峰位置及峰强度与JCPDS卡片(85-1364)中Mg2SiO4的物相一致。
2.1.2 Mg2SiO4的Raman光谱
图3所示为Mg2SiO4在不同温度下的Raman谱。由图3可知,Mg2SiO4在高频区有5个特征峰,分别位于820、856、889、951和997 cm-1,其中820和856 cm-1属于硅氧四面体中非桥氧键的对称伸缩振动,889、951和997 cm-1属于硅氧四面体中非桥氧键的反对称伸缩振动特征峰;中频区700和608 cm-1属于桥氧Si—Obr—Si的反对称变形振动的特征峰;低频区464 cm-1属于硅氧四面体的旋转振动,338和360 cm-1分别归属于Mg—O反对称弯曲振动和弯曲振动[6-8]。Mg2SiO4两个最强谱峰位于856和889 cm-1处,分布在800~900 cm-1之间,表明Mg2SiO4是具有岛状结构的硅酸盐。
由于温度的增加会导致声子激发,声子间相互作用增强,致使拉曼谱带宽化,随着温度的升高,Mg2SiO4所有的拉曼谱带都向低频方向移动。在温度升至400 ℃时,338和360 cm-1谱带由于温度导致的谱带宽化而合并成为一条谱带;温度的增加也会导致晶体膨胀,键长伸长,力常数减小,拉曼振动频率强度减弱,以至消失,当温度升至500 ℃时,700和608 cm-1的谱带消失。
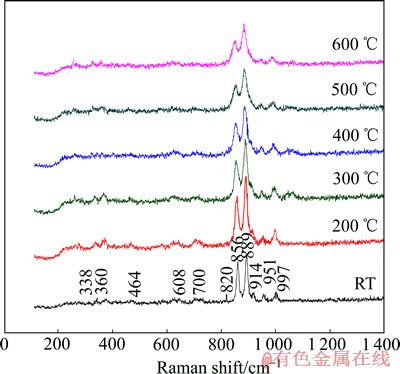
图3 不同温度下Mg2SiO4的Raman谱
Fig. 3 Raman patterns of Mg2SiO4 at different temperatures
2.2 正交试验结果与分析
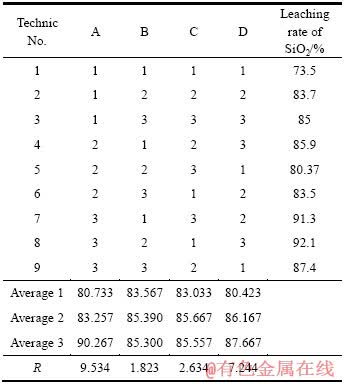
在探索性试验研究的基础上,采用正交表L9(34)设计试验,各因素和水平列于表1。试验结果如表2所列,采取极差法对正交试验结果进行统计分析,由极差R的大小可知:1)在各因素选定的范围内,影响Mg2SiO4中SiO2的浸出率各因素主次关系如下:温度的影响最为显著,其次是时间、NaOH含量和液固比;2)在高NaOH含量体系中,Mg2SiO4中SiO2的浸出率最佳试验条件如下:温度220 ℃,时间120 min,液固比6:1,NaOH溶液含量85%。
表1 Mg2SiO4浸出实验因素水平表
Table 1 Factors and levels of leaching experiment of Mg2SiO4
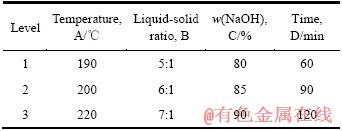
表2 正交试验结果与分析
Table 2 Results and analysis of orthogonal test
2.3 浸出反应机理分析
2.3.1 Mg2SiO4在高NaOH含量体系中浸出反应渣的XRD分析
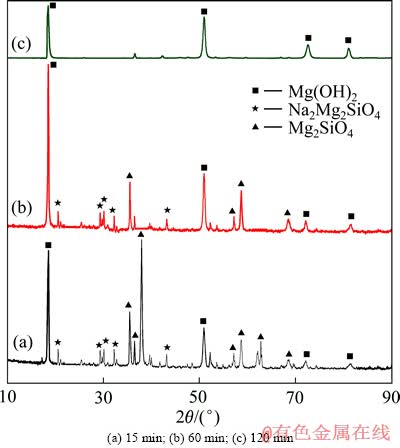
图4 不同反应时间得到渣的XRD谱
Fig. 4 XRD patterns of residue at different reaction times
图4所示为在最佳试验条件下,高NaOH含量中Mg2SiO4浸出反应不同时间所取碱浸水浸渣的XRD谱。由图4可知,反应前15 min,渣相主要为Mg2SiO4、Na2MgSiO4和Mg(OH)2,由于反应渣经过水浸、离心分离处理,且反应中可能生成的Na4SiO4或Na2SiO3易溶于水而进入液相,因此反应渣的XRD谱中未显示Na4SiO4或Na2SiO3的衍射峰。随着反应时间的延长,Mg2SiO4和Na2MgSiO4 的衍射峰强度降低,这是由于Mg2SiO4和Na2MgSiO4与NaOH反应生成Na2MgSiO4和Mg(OH)2。反应120 min后,渣相中只有Mg(OH)2,说明在反应过程中Na+逐步替代Mg2+与硅氧四面体结合,随着反应时间的延长,反应逐渐趋于完全,Mg2+完全被Na+替代,形成Na4SiO4进入液相,而生成的Mg(OH)2进入渣相,根据XRD谱可以推测Mg2SiO4在高NaOH含量体系中主要反应方程式如下:
2Mg2SiO4+6NaOH=Na2MgSiO4+3Mg(OH)2+Na4SiO4 (2)
Mg2SiO4+2NaOH=Na2MgSiO4+Mg(OH)2 (3)
Na2MgSiO4+2NaOH=Na4SiO4+Mg(OH)2 (4)
Mg2SiO4+4NaOH=2Mg(OH)2+Na4SiO4 (5)
2.3.2 Mg2SiO4在高NaOH含量体系中Raman光谱测定
为了进一步了解Mg2SiO4与NaOH的反应过程,采用Raman光谱在线测试整个反应过程。图5所示为在最佳试验条件下Mg2SiO4与NaOH反应随时间变化的拉曼光谱。其中图5(a)所示为拉曼光谱全图、图5(b)和(c)所示为局部放大图,由图5可知,拉曼振动谱峰主要集中在200~4 000 cm-1之间。其中1 074、3 569和3 633 cm-1属于NaOH溶液的特征峰[9];828、856和889 cm-1属于Mg2SiO4的特征峰;由于NaOH对Mg2SiO4谱峰有明显的屏蔽效应,一些Mg2SiO4的特征峰在此温度下无法观测到。为了更直观地分析谱图,把波长在0~1 200 cm-1和3 400~3 700 cm-1范围局部放大。
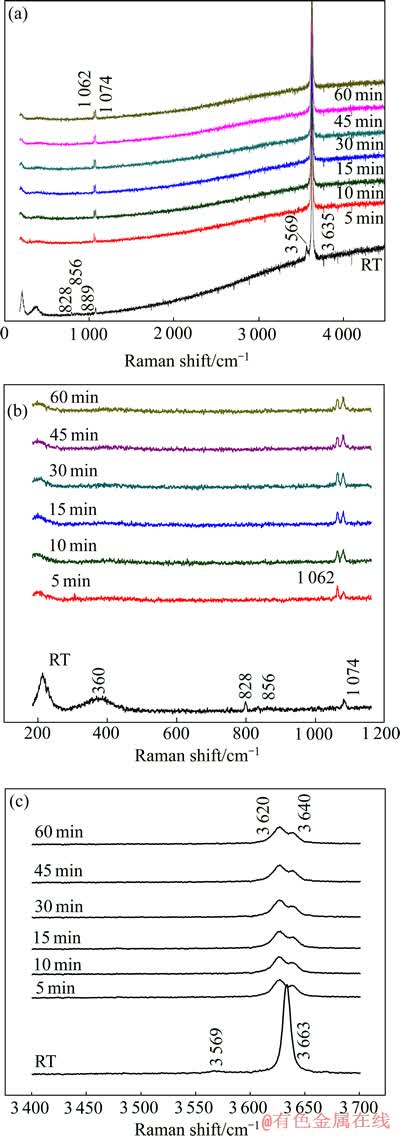
图5 Mg2SiO4在高NaOH含量体系中随时间变化的拉曼光谱及局部放大图
Fig. 5 Raman spectroscopy and local enlarging drawings of Mg2SiO4 at different times in high NaOH content system
由波长在0~1 200 cm-1范围局部放大图(图5(b))可见,随着反应时间延长,200~1 200 cm-1范围内Mg2SiO4位于856、828和889 cm-1处硅氧四面体中非桥氧键的特征峰逐渐向高频移动,400cm-1以下反映固态长程有序的大骨架振动峰逐渐向低频移动且谱峰展宽,说明Mg2SiO4在反应过程中结构发生变化,不在保持长程有序而向无序化转变[10-13]。随着反应时间延长,在965 cm-1处硅氧四面体中非桥养键的特征峰开始显现,逐渐增强,说明非桥氧键反对称伸缩振动峰逐渐增加,反映体系中硅氧四面体从与Mg2+结合转变为与Na+结合,Mg2SiO4经过Na2MgSiO4逐步向Na4SiO4转化的变化过程。体系中NaOH过量,由于模糊效应,谱图中未在965cm-1显示出非桥氧键特征峰。在1 062 cm-1出现特征峰,是体系中硅氧四面体中桥氧键的伸缩振动。
由波长在3 400~3 700 cm-1范围局部放大图可见,随着反应时间延长,体系中NaOH逐渐消耗,3 200~ 3 700 cm-1范围内NaOH溶液特征峰逐渐消失,由于体系中水逐渐减少,3 569 cm-1处H3O2-的振动谱峰逐渐消失,3 633 cm-1处NaOH溶液OH-的伸缩振动峰逐渐向低频移动至3 620 cm-1处,3 640 cm-1处的谱峰开始显现,逐渐增强,并带来右侧峰的展宽,说明体系中Na—O键逐渐被Mg—O键取代, Mg2+经过碱浸过程可以脱离SiO4阵列,以Mg(OH)2形式从其硅酸盐中得以释放。由于在反应过程中同时存在 NaOH和Mg(OH)2,所以在高频区OH的拉曼特征峰出现明显宽化。
4 结论
1) 以Mg(OH)2·4MgCO3·6H2O和SiO2为原料,在1 300 ℃合成的Mg2SiO4是具有Q2结构链状硅酸盐。
2) 通过正交试验得到高浓度NaOH中Mg2SiO4浸出反应最佳试验条件如下:温度220 ℃,时间120 min,液固比6:1,NaOH溶液中NaOH质量分数85%。
3) 高浓度NaOH中Mg2SiO4浸出反应在线拉曼光谱分析显示,Mg2SiO4的岛状结构特征峰在高NaOH含量中随反应的进行逐渐消失,在反应过程中硅氧四面体中的Si—O键被破坏,NaOH介入硅酸盐晶格中,Mg2+经过碱浸过程可以脱离SiO4阵列,以Mg(OH)2形式从其硅酸盐中得以释放。
REFERENCES
[1] 张永禄, 王成彦, 徐志峰. 低品位碱预处理红土镍矿加压浸出[J]. 过程工程学报, 2010, 10(2): 263-269.
ZHANG Yong-lu, WANG Cheng-yan, XU Zhi-feng. Pressure leaching of alkali-pretreated limonitic laterite ore[J]. The Chinese Journal of Process Engineering, 2010, 10(2): 263-269.
[2] 朱景和. 世界镍红土矿资源开发与利用技术分析[J]. 世界有色金属, 2007(10): 7-9.
ZHU Jing-he. Exploration laterite-nickel ore and analysis on utilization technology[J]. World Nonferrous Metals, 2007(10): 7-9.
[3] 刘 燕, 王永志, 陆 雷. 红土镍矿干燥特性的研究[J]. 中国有色冶金, 2010, 39(1): 54-56.
LIU Yan, WANG Yong-zhi, LU Lei. Design of pipeline mixer-settler extractor[J]. China Nonferrous Metallurgy, 2010, 39(1): 54-56.
[4] 李金辉, 周友元, 熊道陵. 硫化法分离红土矿中镍铁的研究[J]. 矿冶工程, 2010, 30(1): 47-50.
LI Jin-hui, ZHOU You-yuan, XIONG Dao-ling. Study on separating nickel and iron in laterite by sulphidizing method[J]. Mining and Metallurgical Engineering, 2010, 30(1): 47-50.
[5] 徐建峰, 石 干, 马明军. MgO加入量和煅烧温度对镁橄榄石材料相组成的影响[J]. 耐火材料, 2008, 42(5): 354-356.
XU Jian-feng, SHI Gan, MA Ming-yu. MgO quantity and calcining temperature of magnesium olivine material composition[J]. Refractories, 2008, 42(5): 354-356.
[6] de OLIVEIRA E F, HASE Y. Infrared study and isotopic effect of magnesium hydroxide[J]. Vibrational Spectroscopy, 2001, 25: 53-56.
[7] TSAI M T. Characterization of nanocrystalline forsterite fiber synthesized via the sol-gel process[J]. J Am Ceram Soc, 2002, 85(2): 453-458.
[8] TANI T, SAEKI S. Chromium-doped forsterite nanoparticle synthesis by flame spray pyrolysis[J]. J Am Ceram Soc, 2007, 85(2): 805-808.
[9] WALRAFEN G E, DOUGLASA R T W. Raman spectra from very concentrated aqueous NaOH and from wet and dry solid and anhydrous molten, LiOH, NaOH, and KOH[J]. J Chem Phys, 2006, 124: 114504-114518.
[10] 王 蓉, 张保民. 辉石的拉曼光谱[J]. 光谱学与光谱分析, 2010, 30(2): 376-380.
WANG Rong, ZHANG Bao-min. Raman spectra of pyroxene[J]. Spectroscopy and Spectral Analysis, 2010, 30(2): 376-380.
[11]  M, PALUSZKIEWICZ C. The structural role of alkaline earth ions in oxyfluoride aluminosilicate glasses-infrared spectroscopy study[J]. Vibrational Spectroscopy, 2008, 48: 246-250.
M, PALUSZKIEWICZ C. The structural role of alkaline earth ions in oxyfluoride aluminosilicate glasses-infrared spectroscopy study[J]. Vibrational Spectroscopy, 2008, 48: 246-250.
[12] 吴永全, 尤静林, 蒋国昌, 陈 辉. CaSiO3从熔体到玻璃的结构和拉曼光谱性质研究[J]. 无机化学学报, 2004, 20(2): 133-138.
WU Yong-quan, YOU Jing-lin, JIANG Guo-chang, CHEN Hui. Theoretical study of structural and Raman spectral properties of CaSiO3 quenched from melt to glass[J]. Chinese Journal of Inorganic Chemistry, 2004, 20(2): 133-138.
[13] LIN Chung-cherng, CHEN Shih-fan, LIU Lin-gun. Anionic structure and elasticity of Na2O-MgO-SiO2 glasses[J]. Journal of Non-Crystalline Solids, 2007, 353: 413-425.
(编辑 龙怀中)
基金项目:国家重点基础研究发展计划资助项目(2007CB613603);上海市现代冶金与材料制备重点实验室开放课题项目(SELF-2009-02)
收稿日期:2012-09-07;修订日期:2013-04-26
通信作者:翟玉春,教授,博士;电话:024-83687731;E-mail: lnzhaochangming@yahoo.com.cn