硅酸盐在(NH4)2WO4-(NH4)2CO3-NH3-H2O体系中反应行为的热力学分析
来源期刊:中国有色金属学报(英文版)2018年第11期
论文作者:李小斌 申雷霆 童潇依 齐天贵 刘桂华 周秋生 彭志宏
文章页码:2342 - 2350
关键词:仲钨酸铵;除硅;镁盐沉淀法;溶液净化
Key words:ammonium paratungstate; silicon removal; magnesium salt precipitation; solution purification
摘 要:含硅组分在(NH4)2WO4-(NH4)2CO3-NH3-H2O体系中的反应行为对仲钨酸铵清洁生产新技术的研发具有重要意义。为了探索此体系中硅的脱除方法,对含硅组分的反应行为进行系统的热力学分析。热力学计算结果表明,钨矿物焙烧熟料中的硅酸盐在浸出过程中会发生部分分解,25 °C条件下溶出体系中硅浓度在热力学上可达到150~160 mg/L。溶液中溶解的硅可采用镁盐沉淀法除去,体系中低碳酸根浓度和高铵浓度有利于硅的脱除,当体系中总碳酸根浓度高于1.5 mol/L时,硅酸镁沉淀难以形成。在合适的除硅条件下,在溶液中添加镁盐,硅浓度可降至8~10 mg/L。此外,研究体系中的钙和镁的反应行为,发现实验结果与理论计算结果一致。
Abstract: The reaction behaviors of silicate species in (NH4)2WO4-(NH4)2CO3-NH3-H2O system are crucial to developing a green manufacture technique for ammonium paratungstate. In order to efficiently remove silicon from the system, the reaction behaviors of silicate species were systematically investigated by thermodynamic analysis. The thermodynamic analysis shows that silicate in the tungstate clinker partly decomposes in the leaching process, with 150-160 mg/L silicon thermodynamically at 25 °C. The dissolved silicon can be removed by magnesium salts via forming insoluble MgSiO3. The low carbonate and high ammonia concentrations in the system are beneficial to the removal of silicon, with silicon concentration reaching 8-10 mg/L thermodynamically, whereas MgSiO3 precipitation is hardly formed when the concentration of total carbonate is more than 1.5 mol/L. The reaction behaviors of calcium and magnesium were also studied in the system. The results in the verification experiments consist with the theoretical calculation.
Trans. Nonferrous Met. Soc. China 28(2018) 2342-2350
Xiao-bin LI, Lei-ting SHEN, Xiao-yi TONG, Tian-gui QI, Gui-hua LIU, Qiu-sheng ZHOU, Zhi-hong PENG
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 7 September 2017; accepted 8 March 2018
Abstract: The reaction behaviors of silicate species in (NH4)2WO4-(NH4)2CO3-NH3-H2O system are crucial to developing a green manufacture technique for ammonium paratungstate. In order to efficiently remove silicon from the system, the reaction behaviors of silicate species were systematically investigated by thermodynamic analysis. The thermodynamic analysis shows that silicate in the tungstate clinker partly decomposes in the leaching process, with 150-160 mg/L silicon thermodynamically at 25 °C. The dissolved silicon can be removed by magnesium salts via forming insoluble MgSiO3. The low carbonate and high ammonia concentrations in the system are beneficial to the removal of silicon, with silicon concentration reaching 8-10 mg/L thermodynamically, whereas MgSiO3 precipitation is hardly formed when the concentration of total carbonate is more than 1.5 mol/L. The reaction behaviors of calcium and magnesium were also studied in the system. The results in the verification experiments consist with the theoretical calculation.
Key words: ammonium paratungstate; silicon removal; magnesium salt precipitation; solution purification
1 Introduction
The tungsten is an important metal in many industrial and military applications due to its properties of high melting point, high hardness, large density and high strength at elevated temperatures [1-4]. To obtain high-purity tungsten products, ammonium paratungstate (APT) acts as an important intermediate product in the process of tungsten metallurgy. The commercial APT manufacture processes are treating tungsten concentrates by soda or caustic soda leaching methods [5,6]. In these processes, the impure sodium tungstate solution is obtained by decomposing tungsten concentrates in soda or caustic soda solutions, and then the sodium tungstate solution goes through purification and conversion stages, finally the APT·4H2O product is obtained by evaporative crystallization [3,7]. However, these conventional methods consume a large amount of auxiliary materials, and the conversion process of sodium tungstate to ammonium tungstate will discharge a mass of high salinity wastewater which is difficult to handle and unable to recycle economically [2,8], resulting in serious environmental pollution [9]. The conventional open- circuit processes for APT production become much harder to meet the increasingly strict environmental requirement nowadays.
In order to alleviate the environmental stresses caused by wastewater discharge in the APT manufacture process, we proposed a novel extractive technology for tungsten metallurgy [10,11]. In this approach, the mixture (raw meal) of tungsten concentrate and calcium carbonate is roasted at 800-850 °C to convert tungsten-bearing minerals into Ca3WO6 [12] and/or Ca2FeWO6 [13], which are readily soluble in aqueous ammonium carbonate solution. Subsequently, the roasted product (clinker) is leached by ammonium carbonate solution at atmospheric pressure to obtain (NH4)2WO4- (NH4)2CO3-NH3 leaching solution and leaching residue of calcium carbonate that is recycled to prepare raw meal. The solution after purification is sent for APT crystallization via evaporation, and the spent solution together with supplementary ammonium carbonate is recycled back to leaching stage to achieve a “closed circulation” for APT production.
The silica-containing minerals, such as quartz, are the common gangues in tungsten concentrates. The reaction behaviors of these silica-containing minerals in the roasting process for tungstate clinker preparation have been studied with the formation of calcium silicates in our previous research [14]. However, LIN and MALTZ [15] suggest that calcium silicates generated at the temperature lower than 1050 °C are unstable in ammonium carbonate solutions. The calcium silicate in the tungstate clinker can be partly decomposed by carbonate solution in the following leaching process, resulting in a high silica concentration in the obtained (NH4)2WO4-(NH4)2CO3-NH3 solution, which may distinctly interfere with the APT product quality. In conventional process, the dissolved silicate can be removed efficiently by adding magnesium salts into sodium or potassium carbonate solutions by forming magnesium silicate precipitate [16], but most of published studies only cover the dissolution and precipitation behaviors of silicates in sodium or potassium carbonate solution. Nevertheless, the feasibility and efficiency of magnesium salts for silicate removal in ammonium carbonate solution have still not been clarified because very few investigations involve the reaction behaviors of silicates in ammonium carbonate leaching solution. Therefore, better understanding of the calcium silicate dissolution and magnesium silicate precipitation in the (NH4)2WO4-(NH4)2CO3-NH3-H2O system is vitally important for product quality control in the new tungsten extraction technology.
In this work, the thermodynamic equilibria of silicate species in (NH4)2WO4-(NH4)2CO3-NH3-H2O system were theoretically calculated at 25 °C to clarify the dissolution and precipitation behaviors of silicates in the system, based on the existing thermodynamic data, the principles of simultaneous equilibrium electronic charge neutrality and mass conservation. The variations of the equilibrium concentrations of Ca2+ and Mg2+ in the system were also calculated for their important effects on the equilibrium concentrations of silicates in the solutions. Furthermore, the verification experiments were carried out to test the reliability of the thermodynamic calculation.
2 Thermodynamic data and calculation model
In the Ca2+-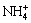 -
- -
-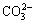 -
-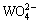 -H2O system, 19 species, i.e. H+, OH-,
-H2O system, 19 species, i.e. H+, OH-, 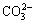 ,
, 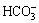 ,
, 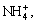 NH3(aq), NH2COO-, Ca2+, Ca(NH3)2+,
NH3(aq), NH2COO-, Ca2+, Ca(NH3)2+,  , Ca(OH)+, Ca(OH)2(aq), CaCO3(aq),
, Ca(OH)+, Ca(OH)2(aq), CaCO3(aq),  ,
, 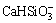 ,
,  ,
, 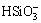 , H2SiO3(aq), and
, H2SiO3(aq), and 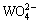 are assumed to exist in the solution. While the possible species in the Mg2+-
are assumed to exist in the solution. While the possible species in the Mg2+-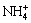 -
- -
-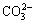 -
-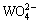 -H2O system are H+, OH-,
-H2O system are H+, OH-, 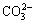 ,
, 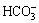 ,
, 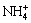 , NH3(aq), NH2COO-, Ca2+, Mg(NH3)2+,
, NH3(aq), NH2COO-, Ca2+, Mg(NH3)2+, 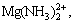
 Mg(OH)+, MgCO3(aq),
Mg(OH)+, MgCO3(aq), 


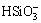 and H2SiO3(aq). The formation of poly-tungstate was ignored in alkaline leaching system. The possible reactions involved in the system and their corresponding equilibrium constants at 25 °C are presented in Table 1. The possible solid phases existing in the system are CaCO3, CaWO4, CaSiO3, H2SiO3, Ca(OH)2 and MgCO3, MgWO4, MgSiO3, H2SiO3, Mg(OH)2, which are determined by the equilibrium of Reactions (15)-(19) and Reactions (27)-(30) in Table 1, respectively.
and H2SiO3(aq). The formation of poly-tungstate was ignored in alkaline leaching system. The possible reactions involved in the system and their corresponding equilibrium constants at 25 °C are presented in Table 1. The possible solid phases existing in the system are CaCO3, CaWO4, CaSiO3, H2SiO3, Ca(OH)2 and MgCO3, MgWO4, MgSiO3, H2SiO3, Mg(OH)2, which are determined by the equilibrium of Reactions (15)-(19) and Reactions (27)-(30) in Table 1, respectively.
Table 1 Solubility and equilibrium reactions in Mg2+/Ca2+- 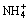 -
-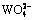 -
- -
-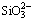 -H2O systems at 25 °C
-H2O systems at 25 °C

The equilibrium concentrations of the species in the complex aqueous system can be calculated based on the three principles of (1) simultaneous equilibrium, (2) electronic charge neutrality, and (3) mass conservation. Considering the lack of the activity coefficient data of the soluble component, the activity was replaced by mole concentration in the model. As the modeling process for Ca2+-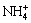 -
- -
-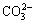 -
- 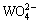 -H2O system is similar to that for Mg2+-
-H2O system is similar to that for Mg2+-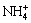 -
-  -
-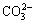 -
-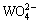 -H2O system, we can hereby make the former system as an example to explain in detail.
-H2O system, we can hereby make the former system as an example to explain in detail.
Firstly, according to the simultaneous equilibrium and stability constants of Reactions (1)-(5) shown in Table 1, the concentrations of the species of OH-, 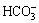 ,
, 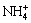 , and NH2COO- in the equilibrium system can be expressed as Eq. (1):
, and NH2COO- in the equilibrium system can be expressed as Eq. (1):
c(i)=fi(c(H+), c(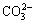 ), c(NH3(aq))) (1)
), c(NH3(aq))) (1)
where c(i) is the mole concentration of specie i, and i is OH-, 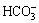 ,
, 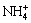 or NH2COO-.
or NH2COO-.
Meanwhile, CaSiO3 and H2SiO3 are the possible silica-containing solid phases which may be simultaneous equilibrium with the calcium-containing solid phases of CaCO3, Ca(OH)2 and/or CaWO4. So, the equilibrium concentrations of  and Ca2+ in the system can be expressed as Eqs. (2) and (3), respectively:
and Ca2+ in the system can be expressed as Eqs. (2) and (3), respectively:
c( )=fj (c(Ca2+) or c(H+)) (2)
)=fj (c(Ca2+) or c(H+)) (2)
c(Ca2+)=fk (c( ) or c(
) or c(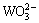 ) or c(OH-)) (3)
) or c(OH-)) (3)
For a certain equilibrium system, the equilibrium solid phases of silica-containing phase and calcium- containing phase are fixed. Under this circumstance, the relationships between c( ), c(Ca2+) c(
), c(Ca2+) c(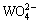 ) and c(H+) or c(
) and c(H+) or c(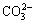 ), as shown in Eq. (4), can be obtained by combining Eqs. (1)-(3).
), as shown in Eq. (4), can be obtained by combining Eqs. (1)-(3).
c(i)=fi (c(H+) or c( )) (4)
)) (4)
where i is  , Ca2+ or
, Ca2+ or 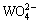 .
.
Similarly, the equation for the concentrations of the remain species, Ca(NH3)2+,  , Ca(OH)+, Ca(OH)2(aq), CaCO3(aq),
, Ca(OH)+, Ca(OH)2(aq), CaCO3(aq),  ,
, 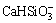 ,
,  ,
, 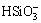 , and H2SiO3(aq), can be obtained as Eq. (5) based on the equilibrium relationships of Reactions (8)-(14):
, and H2SiO3(aq), can be obtained as Eq. (5) based on the equilibrium relationships of Reactions (8)-(14):
c(i)=fi (c(H+), c( ), c(NH3(aq))) (5)
), c(NH3(aq))) (5)
where i is Ca(NH3)2+, Ca(NH3)22+, Ca(OH)+, Ca(OH)2(aq), CaCO3(aq),  ,
, 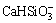 ,
,  ,
, 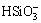 or H2SiO3(aq).
or H2SiO3(aq).
Secondly, according to the mass conservation in the system, Eqs. (6) and (7) can be established from Eqs. (1), (4) and (5).
c(NT)=c(NH4+)+c(NH3(aq))+c(NH2COO-)+c(Ca(NH3)2+)+2c(Ca(NH3)22+)=
fNT (c(H+), c(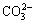 ), c(NH3(aq))) (6)
), c(NH3(aq))) (6)
c(CT)=c(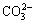 )+c(
)+c(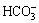 )+c(NH2COO-)+c(CaHCO3+)=fCT (c(H+), c(CO32+), c(NH3(aq)) (7)
)+c(NH2COO-)+c(CaHCO3+)=fCT (c(H+), c(CO32+), c(NH3(aq)) (7)
where c(NT) and c(CT) are total mole concentrations of ammonia and carbonate in the solution, respectively.
Moreover, according to the principle of electronic charge neutrality, the equation of charge equilibrium can be expressed as Eq. (8):
c(H+)+c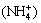 +2c(Ca2+)+2c(Ca(NH3)2+)+
+2c(Ca2+)+2c(Ca(NH3)2+)+
2c +c(Ca(OH)+)+2c(CaHCO3+)=
+c(Ca(OH)+)+2c(CaHCO3+)=
c(OH-)+2c(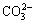 )+c(
)+c(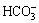 )+c(NH2COO-)+
)+c(NH2COO-)+
c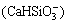 +2c
+2c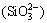 +c
+c +2c
+2c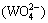 (8)
(8)
Based on Eqs. (1), (4), (5) and (8), another independent equation can be obtained:
f (c(H+), c(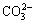 ), c(NH3(aq)))=0 (9)
), c(NH3(aq)))=0 (9)
Finally, the thermodynamic model of the Ca2+- 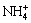 -
- -
-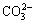 -
-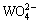 -H2O system can be established by a combination of Eqs. (6)-(8). There are 3 independent equations containing 5 variables, i.e. c(NT), c(CT), c(H+), c(
-H2O system can be established by a combination of Eqs. (6)-(8). There are 3 independent equations containing 5 variables, i.e. c(NT), c(CT), c(H+), c( ) and c(NH3(aq)). Thus, if certain values of c(NT) and c(CT) are given, the other 3 variables of c(H+), c(
) and c(NH3(aq)). Thus, if certain values of c(NT) and c(CT) are given, the other 3 variables of c(H+), c( ) and c(NH3(aq)) can be obtained from the above mentioned simultaneous equations by the computation program established in the Microsoft Excel, based on the Newton-Rephson iteration method. Consequently, the equilibrium concentrations of all the species in the system can be obtained from Eqs. (1)-(5). Considering the solubilities of ammonia, ammonium carbonate and ammonium bicarbonate, the concen- trations of silica-containing species in the system were calculated with c(NT) varying from 0 to 20 mol/L and c(NT) varying from 0-5 mol/L.
) and c(NH3(aq)) can be obtained from the above mentioned simultaneous equations by the computation program established in the Microsoft Excel, based on the Newton-Rephson iteration method. Consequently, the equilibrium concentrations of all the species in the system can be obtained from Eqs. (1)-(5). Considering the solubilities of ammonia, ammonium carbonate and ammonium bicarbonate, the concen- trations of silica-containing species in the system were calculated with c(NT) varying from 0 to 20 mol/L and c(NT) varying from 0-5 mol/L.
3 Results and discussion
3.1 Silicate behavior in (NH4)2WO4-(NH4)2CO3- NH3-H2O system
In the new green manufacture technique, tungsten concentrate is roasted with calcium carbonate to convert tungstate to readily soluble Ca3WO6 [12]. Our previous study [14] has testified the formation of calcium silicates in the roasting process in which the silica-containing minerals readily react with calcium carbonate under the roasting conditions. In view of the fact that CaSiO3 is the most stable calcium silicate phase in the carbonate solutions compared other calcium silicates, and CaCO3 in the form of vaterite (lg K=-7.91) is found as the main phase in the tungsten clinker leaching experiments [24], the stability of CaSiO3 and thermodynamic equilibrium concentration of silicate in the system for tungsten clinker leaching process are analyzed firstly. In the calculation process, the possible equilibrium solid phases of CaSiO3, H2SiO3, CaCO3 and CaWO3 are taken into account, and correspondingly, Reactions (16)-(19) are simultaneous equilibrium.
Figure 1 shows the distribution of equilibrium phases of silicates in the tungsten clinker leaching system. The results indicate that CaSiO3 phase only exists in the solution within very low range of c(CT) (0-0.025 mol/L). Calcium silicate cannot stably exist thermodynamically in the actual tungsten clinker leaching process, in which c(CT) is more than 2 mol/L in order to restrain the secondary reaction between tungstate ions and calcium carbonate [24]. CaSiO3 can be decomposed and converted to H2SiO3 phase when c(CT) increases in the leaching solution.

Fig. 1 Stable phases of silicates in (NH4)2WO4-(NH4)2CO3- NH3-H2O system at 25 °C
Figure 2 illustrates the concentration variation of dissolved silicate in the solution with different c(NT) and c(CT). The result shows that the dissolved silicate in the system is almost constant when c(CT) is higher than 0.5 mol/L (lg c(CT)≈-0.3). Beyond this region, the peak value of the dissolved silicate in the system appears at the equilibrium boundary line for CaSiO3 and H2SiO3. The dissolved silicate content obviously increases with increasing c(NT), and decreases with increasing c(CT) in H2SiO3 stable region. Correspondingly, the dissolved silicate content has a reverse variation trend with c(NT) and c(CT) in CaSiO3 stable region.
Figure 3 presents the influence of c(NT) and c(CT) on the distribution of the dissolved silicate species. The results indicate that the mole fractions of dissolved silicate species, 
 H2SiO3(aq) and
H2SiO3(aq) and 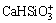 , have different behaviors with the changes of c(NT) and c(CT) in the solution system.
, have different behaviors with the changes of c(NT) and c(CT) in the solution system. 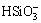 is the primary dissolved silicate species in the solution system in high c(NT) and low c(CT) region, while H2SiO3(aq) gradually becomes the dominant silica-containing species when decreasing c(NT) and/or rising c(CT). Both
is the primary dissolved silicate species in the solution system in high c(NT) and low c(CT) region, while H2SiO3(aq) gradually becomes the dominant silica-containing species when decreasing c(NT) and/or rising c(CT). Both  and
and 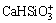 are of small fractions in dissolved silicate species, and their mole fractions are smaller than 10% in the system. It is noteworthy that all of the dissolved silicate species equilibrate with each other simultaneously as shown in Table 1, and any change of the species can make a new equilibrium and redistribution of the species. This is the thermodynamic basis for silicon removal in the solution systems.
are of small fractions in dissolved silicate species, and their mole fractions are smaller than 10% in the system. It is noteworthy that all of the dissolved silicate species equilibrate with each other simultaneously as shown in Table 1, and any change of the species can make a new equilibrium and redistribution of the species. This is the thermodynamic basis for silicon removal in the solution systems.
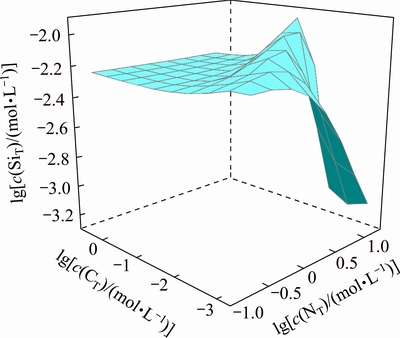
Fig. 2 Relationship among c(SiT), c(NT) and c(CT) in Ca2+-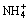 -
-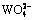 -
- -
-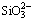 -H2O system at 25 °C
-H2O system at 25 °C
According to the simultaneous equilibrium of CaWO3 and CaCO3, the variation of equilibrium concentrations of tungstate  and dissolved calcium c(CaT) with the change of c(NT) and c(CT) in the leaching system are shown in Figs. 4 and 5, respectively. The results show that the equilibrium tungstate concentration increases with the increase of c(NT) and c(CT), while the dissolved calcium reaches the peak at c(NT)=20 mol/L and c(CT)=0 mol/L. In practice, the primary goal for the leaching stage is to obtain a high concentration of ammonium tungstate solution (c
and dissolved calcium c(CaT) with the change of c(NT) and c(CT) in the leaching system are shown in Figs. 4 and 5, respectively. The results show that the equilibrium tungstate concentration increases with the increase of c(NT) and c(CT), while the dissolved calcium reaches the peak at c(NT)=20 mol/L and c(CT)=0 mol/L. In practice, the primary goal for the leaching stage is to obtain a high concentration of ammonium tungstate solution (c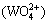 >0.4 mol/L) and keep low impurity levels of dissolved silicate and calcium in the leachate. Based on the results of Figs. 2, 4 and 5, the optimum lixiviant concentrations are c(NT)=10 mol/L and c(CT)=2 mol/L. Under these conditions, the dissolved silicate and calcium in the leachate are estimated at 150-160 and 1-2 mg/L, respectively.
>0.4 mol/L) and keep low impurity levels of dissolved silicate and calcium in the leachate. Based on the results of Figs. 2, 4 and 5, the optimum lixiviant concentrations are c(NT)=10 mol/L and c(CT)=2 mol/L. Under these conditions, the dissolved silicate and calcium in the leachate are estimated at 150-160 and 1-2 mg/L, respectively.
3.2 Effect of c(NT) and c(CT) on silicon concentration

Fig. 3 Distributions of various silicate species with varying c(NT) and c(CT) at 25 °C

Fig. 4 Relationship among c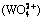 , c(NT) and c(CT) in Ca2+-
, c(NT) and c(CT) in Ca2+-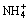 -
-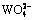 -
- -
-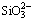 -H2O system at 25 °C
-H2O system at 25 °C
The leachate obtained from the tungsten clinker leaching process contains ammonium tungstate (>0.4 mol/L), high concentrations of ammonium carbonate and free ammonia, and certain contents of the dissolved silicates and calcium (150-160 and 1-2 mg/L, respectively). Before the APT crystallization, most of the ammonium carbonate and free ammonia in the leachate should be distilled and removed from the solution according to the above mentioned new green process. In the ammonium distillation process, the dissolved silicate and calcium may precipitate as H2SiO3 and CaWO4 solids due to the decreased c(NT) and c(CT), thus reducing the impurity levels of the ammonium tungstate solution in the new green process.
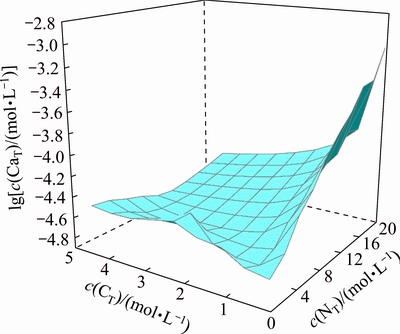
Fig. 5 Relationship among lg c(CaT), c(NT) and c(CT) in Ca2+-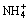 -
-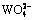 -
- -
-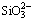 -H2O system at 25 °C
-H2O system at 25 °C
To illustrate the removal of dissolved silica and calcium in the ammonia distillation stage, the variations of their equilibrium concentrations with various c(NT) and c(CT) have been simulated at a constant concentration of c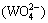 =0.5 mol/L. Figure 6 shows the concentration variation of dissolved silicate with various c(NT) and c(CT) at 25 °C. It is observed that the equilibrium concentration of silicate in the solution is slightly reduced with the reduction of c(NT) in leachate, indicating that a part of dissolved silicate can precipitate from the solution during the ammonia distillation stage. Figure 7 presents the variation of dissolved calcium in the solution with various c(NT) and c(CT), showing that the dissolved calcium in solution drops with the reduction of c(NT). When most of the excess ammonia and ammonium carbonate in the solution evaporate out the concentration of dissolved calcium in the ammonium tungstate solution decreases by one or two orders of magnitude with the precipitation of calcium tungstate. This suggests that the dissolved calcium in ammonium tungstate can be easily removed in ammonia distillation stage.
=0.5 mol/L. Figure 6 shows the concentration variation of dissolved silicate with various c(NT) and c(CT) at 25 °C. It is observed that the equilibrium concentration of silicate in the solution is slightly reduced with the reduction of c(NT) in leachate, indicating that a part of dissolved silicate can precipitate from the solution during the ammonia distillation stage. Figure 7 presents the variation of dissolved calcium in the solution with various c(NT) and c(CT), showing that the dissolved calcium in solution drops with the reduction of c(NT). When most of the excess ammonia and ammonium carbonate in the solution evaporate out the concentration of dissolved calcium in the ammonium tungstate solution decreases by one or two orders of magnitude with the precipitation of calcium tungstate. This suggests that the dissolved calcium in ammonium tungstate can be easily removed in ammonia distillation stage.
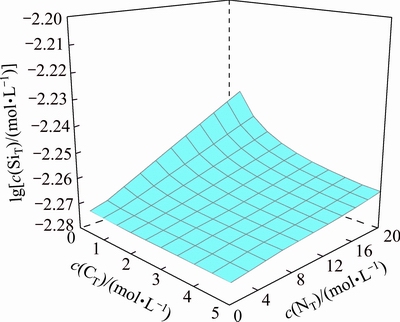
Fig. 6 Distribution of silica concentration in ammonia distillation stage at 25 °C and c(WO3)=0.5 mol/L
Fig. 7 Distribution of calcium concentration in ammonia distillation stage at 25 °C and c(WO3)=0.5 mol/L
3.3 Silicate removal from leachate
Magnesium salt precipitation method is widely used in removing impurities from sodium tungstate and sodium molybdate solutions [25-29]. To verify the effectiveness and the valid conditions in ammonium tungstate solution, the thermodynamic equilibrium concentration of dissolved silicate in ammonium tungstate solution in the presence of magnesium ions was calculated.
When the magnesium salts are added into the silicate-containing ammonium tungstate solution with ammonium carbonate, the magnesium-containing solid phases of MgWO4, MgCO3, MgSiO3, Mg(OH)2 and the silicate-containing solid phases of MgSiO3 and H2SiO3 can form. Based on the simultaneous equilibrium principles, the stable regions for the possible equilibrium solid phases in Mg2+-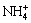 -
-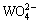 -
-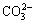 -
- - H2O system are calculated based on the simultaneous equilibrium of Reactions (27)-(30). Figure 8 demonstrates that the solid precipitation of MgSiO3 can form in the solution in low c(CT) (<1.5 mol/L) and high c(NT) (>5 mol/L) regions. H2SiO3 becomes the equilibrium solid phase in the solution at high c(CT), and the magnesium-containing solid phase is MgWO4 in the solution in low c(CT) (<0.5 mol/L) and low c(NT) (<5 mol/L) region.
- H2O system are calculated based on the simultaneous equilibrium of Reactions (27)-(30). Figure 8 demonstrates that the solid precipitation of MgSiO3 can form in the solution in low c(CT) (<1.5 mol/L) and high c(NT) (>5 mol/L) regions. H2SiO3 becomes the equilibrium solid phase in the solution at high c(CT), and the magnesium-containing solid phase is MgWO4 in the solution in low c(CT) (<0.5 mol/L) and low c(NT) (<5 mol/L) region.
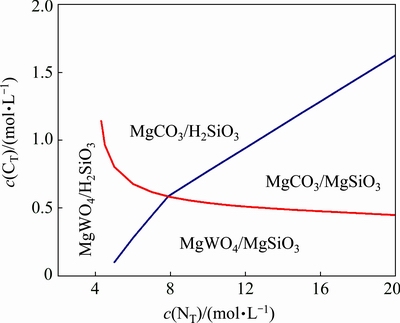
Fig. 8 Relationships among equilibrium solid phases in Mg2+-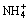 -
-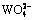 -
- -
-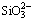 -H2O system at 25 °C and c(WO3)=0.5 mol/L
-H2O system at 25 °C and c(WO3)=0.5 mol/L
The variation of the equilibrium concentration of dissolved silicate with c(CT) and c(NT) in the solution is shown in Fig. 9. It presents that the silicate equilibrium concentration is almost constant at a high value in the solution within a relative high c(CT) region (0.5-5 mol/L). While the concentration of dissolved silica drops dramatically by more than one order of magnitude when the c(CT) reduces to 0.5 mol/L. The boundary line for the significant decrease of the silicate equilibrium concentration corresponds to the equilibrium line for the equilibrium solid phases which contain MgSiO3 (on right side of blue line in Fig. 8). To obtain a high silicate removal percentage, it is necessary to reduce c(CT) as low as possible and keep c(NT)>5 mol/L and thus make the solution composition locked in the regions for the precipitation of MgSiO3. Therefore, magnesium salt precipitation operation used for silicate removal is placed at the end of ammonia distillation stage, where most of the ammonium carbonate in the solution has been distilled out while part of free ammonia is still in the solution [27].
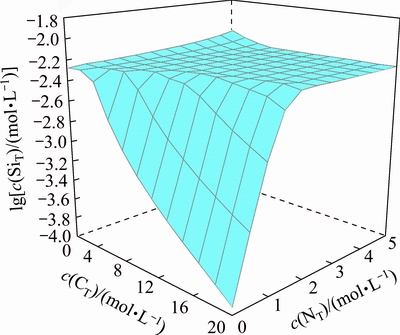
Fig. 9 Distribution of silica concentration in silica removal process at 25 °C and c(WO3)=0.5 mol/L
Figure 10 presents the equilibrium concentration of dissolved magnesium c(MgT) in the ammonium tungstate solution with various c(NT) and c(CT). In general, the dissolved magnesium in the ammonium tungstate solution decreases with the reduction of c(NT). Compared with Fig. 8, MgWO4 is the equilibrium solid phase in the solution in low c(NT) and low c(CT) regions. This means that after the silicate removal operation, the excess magnesium ions can precipitate when the ammonia concentration in the solution reduces in the following APT crystallization operation. To avoid the MgWO4 contamination to APT product in the APT crystallization process, another deep ammonia distillation operation is carried out to precipitate the dissolved magnesium ions. Combining the results in Figs. 9 and 10, this deep ammonia distillation process for magnesium removal is placed at the end of the removal of dissolved silicate.
According to the above thermodynamic calculation results, we can propose a principle flowsheet for the new green technique for APT production as follows: (1) leaching the tungsten clinker in the ammonium carbonate solution with high c(NT) (≥10 mol/L) and c(CT) (≥2 mol/L) to obtain a high c(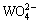 ) ammonium tungstate solution; (2) heating the leachate ammonium tungstate solution to distill most of the ammonium carbonate to make c(CT)<0.5 mol/L in the solution; (3) adding magnesium salts to precipitate the dissolved silicate in the solution and then separate the silicate slags; (4) deep distilling the silicate removed ammonium tungstate solution to precipitate and separate the exceeding dissolved magnesium as magnesium tungstate; (5) evaporating the purified solution to crystallize APT.
) ammonium tungstate solution; (2) heating the leachate ammonium tungstate solution to distill most of the ammonium carbonate to make c(CT)<0.5 mol/L in the solution; (3) adding magnesium salts to precipitate the dissolved silicate in the solution and then separate the silicate slags; (4) deep distilling the silicate removed ammonium tungstate solution to precipitate and separate the exceeding dissolved magnesium as magnesium tungstate; (5) evaporating the purified solution to crystallize APT.
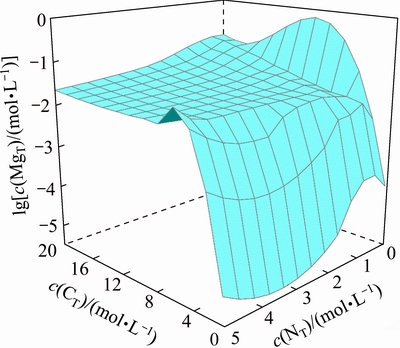
Fig. 10 Distribution of magnesium concentration in silica removal process at 25 °C and c(WO3)=0.5 mol/L
3.4 Experimental verification
To verify the aforementioned thermodynamic calculation results, the tungsten clinker (prepared by the roasting method in Ref. [12] from scheelite) leaching experiments and the silicate removal experiments for the obtained leachate were conducted under various conditions. The chemical compositions of the tungsten clinker are 42.21% WO3, 27.24% Ca, 4.46% Fe and 0.63% Si. The tungsten clinker was added to the aqueous solution of ammonia and ammonium carbonate at a certain concentration, and then agitated at 25 °C for 6 h. The concentrations of tungstate, total ammonia, total carbonate and dissolved silicate in the filtered solution were analyzed by ICP-AES (Thermo Electron Corporation, USA). Silicate removal experiments of the obtained ammonium tungstate solution with and without distilling ammonium carbonate were also conducted by adding sufficient magnesium sulfate. The experimental and thermodynamically calculated values of the silicon concentrations are listed in Table 2.
From Table 2, it can be seen that the experimental and thermodynamically calculated values of silicate concentrations are very similar under the same condition. This indicates that the thermodynamic calculation method and the calculated results are basically correct. The calculated rules of the silicate variation are verified by the experimental results, though the absolute errors of the experimental and thermodynamically calculated silicate concentrations exist according to the results in Table 2. The errors may be caused by the variation of the activity coefficients in the actual solutions. Additionally, the concentrations of calcium and magnesium in some tests were also detected and listed in Table 2, which are consistent with the calculated values. The thermodynamic calculation results provide a theoretical foundation for the impurities removal of the ammonium tungstate solutions.
Table 2 Experimental and theoretical calculation results in verification experiments

4 Conclusions
1) Calcium silicate in the tungsten clinker is not stable in leaching system of (NH4)2WO4-(NH4)2CO3- NH3-H2O. The thermodynamic equilibrium concen- tration of silicon can reach 5.6×10-3 mol/L with H2SiO3 as equilibrium solid phase. The dissolved silicon content increases with increasing ammonia concentration, while it decreases with increasing ammonium carbonate concentration.
2) In ammonia distillation stage, ammonia and ammonium carbonate concentrations have little effect on the equilibrium concentration of silicate in the solution of (NH4)2WO4-(NH4)2CO3-NH3-H2O. Decreasing the total ammonia concentration in the solution is beneficial to the removal of dissolved calcium ion.
3) The dissolved silicate can be easily precipitated by magnesium salts in the ammonium tungstate solution with low carbonate concentration. The concentrations of ammonium carbonate and ammonia in the ammonium tungstate solution obviously influence the efficiency of silicate removal. To obtain the expected silicate removal result, the silicate removal process should be carried out in the solution with high ammonia and low carbonate concentrations.
References
[1] LASSNER E, SCHUBERT W, LUDERITZ E, WOLF H U. Ullmann’s encyclopedia of industrial chemistry [M]. Weinheim: Wiley-VCH, 2000.
[2] LASSBER E, SCHUBERT W D. Tungsten: Properties, chemistry, technology of the element, alloys and chemical compounds [M]. Berlin: Springer, 2011.
[3] YIH S W H, WANG C T. Tungsten: Sources, metallurgy, properties, and applications [M]. New York: Plenum Press, 1979.
[4] HASANABADI M, SHAMSIPUR A, SANI H N, OMIDVAR H, SAKHAEI S. Interfacial microstructure and mechanical properties of tungsten carbide brazed joints using Ag-Cu-Zn+Ni/Mn filler alloy [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 2638-2646.
[5] QUENEAU P B, HUGGINS D K, BECHSTEAD L W. Autoclave soda digestion of refractory scheelite concentrates: US patent, 4320095 [P]. 1982-03-16.
[6] FORWARD F A, VIZSOLYI A I. Process for the production of tungstic acid: US patent, 3193347 [P]. 1965-07-06.
[7] ZHAO Z W, YANG M F, HE L H, ZHANG J L, CHEN X Y, LIU X H. Preparation of Na specific absorbent and application of sodium removal from ammonium tungstate solution [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 854-860.
[8] GAUR R P S. Modern hydrometallurgical production methods for tungsten [J]. Journal of the Minerals Metals & Materials Society (JOM), 2006, 58(58): 45-49.
[9] LIU Liang, XUE Ji-lai. A green leaching method of decomposing synthetic CaWO4 by HCl-H3PO4 in tungsten producing process [C]// Advances in Materials Science for Environmental and Energy Technologies. New Jersey: John Wiley & Sons, Inc, 2010: 157-165.
[10] LI Xiao-bin, CUI Yuan-fa, ZHOU Qiu-sheng, LI Jian-pu, QI Tian-gui, XU Shuang, LIU Gui-hua, LIN Guo-rong, PENG Zhi-hong, LI Ji-hong, XU Xiang-ming, SHEN Lei-ting. A pretreatment method of tungsten raw material: CN patent, 201410527644.6 [P]. 2014-10-9. (in Chinese)
[11] LI Xiao-bin, CUI Yuan-fa, ZHOU Qiu-sheng, LI Jian-pu, QI Tian-gui, XU Shuang. A system for preparing APT by tungsten raw material with zero discharge of waste water: CN patent, 201410528246.6 [P]. 2014-10-9. (in Chinese)
[12] LI Xiao-bin, XU Xiang-ming, ZHOU Qiu-sheng, QI Tian-gui, LIU Gui-hua, PENG Zhi-hong, CUI Yuan-fa, LI Jian-pu. Ca3WO6 prepared by roasting tungsten-containing materials and its leaching performance [J]. International Journal of Refractory Metals & Hard Materials, 2015, 52: 151-158.
[13] LI Xiao-bin, XU Xiang-ming, XU Wang, ZHOU Qiu-sheng, QI Tian-gui, LIU Gui-hua, PENG Zhi-hong, CUI Yuan-fa, LI Jian-pu. Ca3-x(Fe,Mn)xWO6 (0 [14] LI Xiao-bin, XU Xiang-ming, ZHOU Qiu-sheng, QI Tian-gui, LIU Gui-hua, PENG Zhi-hong, CUI Yuan-fa, LI Jian-pu. Thermodynamic and XRD analysis of reaction behaviors of gangue minerals in roasting mixture of scheelite and calcium carbonate for Ca3WO6 preparation [J]. International Journal of Refractory Metals & Hard Materials, 2016, 60: 82-91. [15] LIN I J, MALTZ N S. Alpha-dicalcium silicate formation and the special features of its hydration and interaction with aluminate solution [J]. Journal of Materials Synthesis and Processing, 1997, 5(6): 411-418. [16] ZHAO You-cai, CHEN Jia-yong. Extraction of phosphorus, arsenic and/or silica from sodium tungstate or molybdate solutions with primary amine and tributyl phosphate as solvents. 2: Mechanism of extraction of phosphorus, arsenic and silica from tungstate and molybdate solutions [J]. Hydrometallurgy, 1996, 42(3): 325-335. [17] SPEIGHT J G. Lange’s handbook of chemistry [M]. 16th ed. New York: McGraw-Hill, 2004. [18] MICHALOWSKI T, PIETRZYK A. A thermodynamic study of struvite + water system [J]. Talanta, 2006, 68(3): 594-601. [19] ZHAO Qing, WANG Shu-juan, QIN Feng, CHEN Chang-he. Composition analysis of CO2-NH3-H2O system based on Raman spectra [J]. Industrial & Engineering Chemistry Research, 2011, 50(9): 5316-5325. [20] WAGMAN D D, EVANS W H, PARKER V B, SCHUMM R H, HALOW I, BAILEY S M, CHURNEY K L, NUTTALL R L. The NBS tables of chemical thermodynamic properties: Selected values for inorganic and C1 and C2 organic substances in SI units [J]. Journal of Physical and Chemical Reference Data, 1989, 18(4): 1807-1812. [21] HUMMEL W, BERNER U, CURTI E, PEARSON F J, THOENEN T. Nagra/PSI chemical thermodynamic data base 01/01 [J]. Radiochimica Acta, 2002, 90(9-11): 805-813. [22] ZHANG Bao-ping, TANG Chao-bo, TANG Mo-tang. Dissolution thermodynamic analysis of calcium and magnesium in Mg(II)- Ca(II)-NH3- [23] PLUMMER L N, BUSENBERG E. The solubilities of calcite, aragonite and vaterite in CO2-H2O solutions between 0 and 90 °C, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O [J]. Geochimica et Cosmochimica Acta, 1982, 46(6): 1011-1040. [24] XU Xiang-ming. Theory and technology for directly preparation of (NH4)2WO4 solution by conversion of tungsten-containing materials together with low-temperature leaching in (NH4)2CO3 solution [D]. Changsha: Central South University, 2016. (in Chinese) [25] QUENEAU R B, BECKSTEAD L W, MUGGINS O K. Treatment of sodium tungsten leach liquor containing dissolved silica, phosphorus, and fluorine impurities: US patent, 4311679 [P]. 1982-01-19. [26] HE Gui-xiang, HE Li-hua, ZHAO Zhong-wei, CHEN Xing-yu, GAO Li-li, LIU Xu-heng. Thermodynamic study on phosphorus removal from tungstate solution via magnesium salt precipitation method [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 3440-3447. [27] YANG Liang, ZHAO Zhong-wei, HE Li-hua, HUO Guang-sheng, CHEN Ai-liang. Thermodynamics analysis of arsenic removal by magnesium salt precipitation in ammonium molybdate solution [J]. Journal of Central South University: Science and Technology, 2012, 43(5): 21-26. (in Chinese) [28] PARK Y Y, TRAN T, LEE Y H, NAM Y I, SENANAYAKE G, KIM M J. Selective removal of arsenic (V) from a molybdate plant liquor by precipitation of magnesium arsenate [J]. Hydrometallurgy, 2010, 104(2): 290-297. [29] LASSNER E. From tungsten concentrates and scrap to highly pure ammonium paratungstate (APT) [J]. Hydrometallurgy, 1995, 13: 35-44. 李小斌,申雷霆,童潇依,齐天贵,刘桂华,周秋生,彭志宏 中南大学 冶金与环境学院,长沙 410083 摘 要:含硅组分在(NH4)2WO4-(NH4)2CO3-NH3-H2O体系中的反应行为对仲钨酸铵清洁生产新技术的研发具有重要意义。为了探索此体系中硅的脱除方法,对含硅组分的反应行为进行系统的热力学分析。热力学计算结果表明,钨矿物焙烧熟料中的硅酸盐在浸出过程中会发生部分分解,25 °C条件下溶出体系中硅浓度在热力学上可达到150~160 mg/L。溶液中溶解的硅可采用镁盐沉淀法除去,体系中低碳酸根浓度和高铵浓度有利于硅的脱除,当体系中总碳酸根浓度高于1.5 mol/L时,硅酸镁沉淀难以形成。在合适的除硅条件下,在溶液中添加镁盐,硅浓度可降至8~10 mg/L。此外,研究体系中的钙和镁的反应行为,发现实验结果与理论计算结果一致。 关键词:仲钨酸铵;除硅;镁盐沉淀法;溶液净化 (Edited by Wei-ping CHEN) Foundation item: Project (51274243) supported by the National Natural Science Foundation of China; Project (2015CX001) supported by the Innovation- driven Plan of Central South University, China Corresponding author: Tian-gui QI; Tel/Fax: +86-731-88830453; E-mail: qitiangui@csu.edu.cn DOI: 10.1016/S1003-6326(18)64879-4 -
- -H2O system [J]. Hydrometallurgy of China, 2005, 24(1): 26-31. (in Chinese)
-H2O system [J]. Hydrometallurgy of China, 2005, 24(1): 26-31. (in Chinese)硅酸盐在(NH4)2WO4-(NH4)2CO3-NH3-H2O体系中反应行为的热力学分析

