


Trans. Nonferrous Met. Soc. China 22(2012) 1432-1436
Thermodynamic assessment of Au-Pt system
XU Xiao-ning1, REN Yu-ping1, LI Chang-fa2, LI Song1, QIN Gao-wu1
1. Key Laboratory for Anisotropy and Texture of Materials of Ministry of Education,
Northeastern University, Shenyang 110819, China;
2. School of Materials Science & Engineering, Tianjin University of Technology, Tianjin 300384, China
Received 22 June 2011; accepted 2 September 2011
Abstract: The thermodynamic re-assessment of Au-Pt binary system was carried out by using the Calphad method and based on the recent experimental data. The Gibbs energies of face-centred cubic and liquid phases were described by a sub-regular solution model with the Redlich-Kister equation. Much effort was taken to reproduce the phase equilibrium results and thermodynamic properties of the solid phase, including the activity and mixing enthalpy. The constraint of the third law of thermodynamics was also considered in the assessment. According to the presently assessed results, the miscibility gap region in the Au-Pt system slightly shifts to the Au-rich side, and the critical point of the miscibility gap is about 1200 ℃ and Au-56% Pt.
Key words: Au-Pt system; phase diagram; thermodynamic assessment; Calphad method
1 Introduction
Recently, intense research has been focused on the Au-Pt and Au-Pt based systems because these alloys exhibit unusual mechanical properties, physical properties, chemical stability and biological compatibility and have many potential applications in the fields of electronics, medicines, microelectronic packaging, etc [1-6]. The Au-Pt system is a typical representative of the miscibility gap (MG) occurring in the solid state [7]. Much effort has been focused on the control of the miscibility limit of Au and Pt in the Au-Pt system in order to achieve desirable properties by means of heat treatment and/or the third element addition [1, 8,9]. However, due to the two noble metals Au and Pt employed, it is restricted and delayed to develop the new Au-Pt and Au-Pt based alloys through a trial-and-error method. There is thus an urgent need to adopt a new approach to design and develop the new Au-Pt and Au-Pt based alloys, such as calculation of phase diagrams method (CALPHAD). Unfortunately, the current thermodynamic description of the Au-Pt system can not meet the requirements of the alloy design due to its unreasonable deviation from the practical case.
According to the literature review, there have been two published assessments of the binary Au-Pt system so far. The results assessed and available data are shown in Fig. 1 [7,8,10-19]. The Au-Pt system was firstly assessed by OKAMOTO and MASSALSKI [7] who referred to a compilation of previous data by X-ray diffraction, thermodynamic analysis and electrical resistivity measurement [8,10-17]. However, these data above measured about 50 years ago have some experimental errors inherited from the techniques themselves, and have some differences from our recent phase equilibrium data using the diffusion couple technique [20]. Later, GROLIER and RAINER [18] re-assessed the Au-Pt system using simple sub-regular solution model to the liquid and face-centred cubic (FCC) phases on the basis of these previous experimental data [8,10-15,19]. Their thermodynamic parameters can reproduce most thermodynamic data and phase equilibrium data. However, if the MG of the Au-Pt system is extrapolated to 0 K using the thermodynamic parameters from GROLIER and RAINER [18], as shown in Fig. 1 [7,8,10-19], the Au-rich phase contains about 3%Pt, which appears inconsistent with the third law of thermodynamics. Therefore, the Au-Pt system needs to be further investigated to get one set of reasonable thermodynamic parameters to develop new Au-Pt-based bulk and nanometer materials.

Fig. 1 Present accepted binary Au-Pt phase diagram calculated from available data [7,8,10-19], where circled region indicates Au phase is not pure Au at 0 K
Recently, the MG of the Au-Pt system by diffusion couple technique was measured [20] and it was found that the MG boundary has some deviation from the early data. In this work, the Au-Pt system by means of the CALPHAD method was re-assessed considering our recent experimental MG data [20] and thermodynamic properties of the solid phase, including activity [21] and mixing enthalpy [22,23]. Moreover, the thermodynamic parameters of liquid phase were obtained by optimizing the experimental data on the liquidus and solidus [8,10,11,14]. Finally, a set of self-consistent thermodynamic parameters for each phase were obtained.
2 Experimental
2.1 Phase diagram data
The phase equilibrium of Au-Pt system has been extensively investigated over the last century [7,8, 10-17,19]. The liquidus and solidus of the Au-Pt system were determined by several researchers using thermal analysis on cooling [8,10,11], electrical resistivity [11,14] and microscopy [8]. Most results generally coincide with each other. Large experimental scatters can be observed in the data by GRIGORJEW [10] and GRUBE et al [11]. GRIGORJEW [10] reported a peritectic reaction varying from 1260 to 1300 ℃ and GRUBE et al [11] from 1295 to 1302 ℃. The deviation may be attributed to the adopted measurement method, thermal analysis on cooling, which is always accompanied by supercooling effects. These inaccuracy data were not used in the present study.
The MG in the solid phase has been investigated by XRD [8,12,13,16,17,19], electrical resistivity measure- ments [8,12,14,15] and present diffusion couple method [20]. The results by XRD and resistivity measurement are shown in Fig. 1 [7,8,10-19]. It is obvious that the MG measured by XRD shifts to the Pt-rich side compared with that determined by electrical resistivity. The deviation may depend on the adopted investigation method. For electrical resistivity measurement, RUDNITSKII [24] indicated that the temperature dependence behaviors of the electrical resistances of the alloys on heating were not reproduced on cooling. This indicates the resistivity measurement does not meet the requirement of phase equilibria determination. Moreover, JOHANSSON and HAGSTEN [25] found that a hysteresis effect existed in the resistivity measurements for the Au-Pt alloys, which possibly resulted in some errors in determining the equilibrium phase compositions at a given temperature. For the XRD measurement, the lattice parameters of the solid phase were calculated according to the Vegard’s law and thus the compositions of the corresponding phases were determined based on the hypothesis that annealing temperature seemed to have little effect on the lattice parameters [7]. But according to the results reported by VESNIN and SHUBIN [19] using high temperature X-ray diffraction, the hypothesis was not supported. The results indicated that the lattice parameters for Au-Pt system increased with increasing temperature. Based on the unreasonable hypothesis, the temperature effect on the lattice and strain existed in solid phases of the alloys was overlooked in the process of lattice constant calculation, especially for an insufficient solution treatments and/or aging duration. Therefore, the MG of the Au-Pt system determined by XRD is in some error and shifts to the Pt-rich side. In contrast, the diffusion couple technique used can produce equilibrium phases in bulk, in particular, the equilibrated phase interface is usually a large angle phase boundary with a less strain effect. At the same time, electron probe microanalysis (EPMA) can provide a more accurate composition analysis and finally a more reliable MG can be determined. Therefore, the present assessment was based on the MG reported by diffusion couples method [20], and the data from XRD and electrical resistivity was not used.
2.2 Thermodynamic data
The thermodynamic data of the Au-Pt system is scarce. To the best of our knowledge, no thermodynamic data for the liquid phase has been measured, thus it is difficult to accurately assess the parameters of the liquid phase. While for the solid phases of the Au-Pt system, JONES et al [21] determined the activity of Au in the solid phase at 1100, 1150 and 1200 ℃ by mass spectrometry (Knudsen-cell), and using these data, the formation enthalpy of the solid phase was also calculated. KUBASCHEWSKI et al [22], SCHALLER [23] and De BOER et al [26] reported the formation enthalpy of the solid phase over the entire compositional range at 25 ℃, with solid Au and Pt as reference state. The values showed that the enthalpy of formation is positive and compositional asymmetry. The semi-empirical results of BOER et al [26] showed a large deviation from the other results. Thereby, the results from KUBASCHEWSKI et al [22] and SCHALLER [23] were used in the present work.
3 Thermodynamic modeling and optimization
3.1 Modeling
There are only two phases in the Au-Pt system, liquid and FCC phases. They are described by the sub-regular solution model, according to which the molar Gibbs free energy of α phase (liquid, FCC) is expressed as:
 (1)
(1)
where 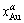 and
and  denote the molar fractions of Au and Pt in the α phase, respectively;
denote the molar fractions of Au and Pt in the α phase, respectively;  and
and 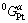 are the molar Gibbs free energies of pure Au and Pt, respectively; and
are the molar Gibbs free energies of pure Au and Pt, respectively; and 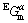 is the excess Gibbs free energy, expressed as a Redlich-Kister polynomial:
is the excess Gibbs free energy, expressed as a Redlich-Kister polynomial:
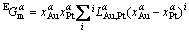 (2)
(2)
where  is the binary interaction parameter between Au and Pt and is temperature dependent as:
is the binary interaction parameter between Au and Pt and is temperature dependent as:
 (3)
(3)
where  and
and  are the constants to be optimized and i is the series. It should be mentioned that, regarding liquid phase, i=0 for simplicity, particularly for its extrapolation to a high-order system. As those investigations [21-23, 26] shown, the formation enthalpy of solid phase is not symmetrical. To describe the composition dependence,
are the constants to be optimized and i is the series. It should be mentioned that, regarding liquid phase, i=0 for simplicity, particularly for its extrapolation to a high-order system. As those investigations [21-23, 26] shown, the formation enthalpy of solid phase is not symmetrical. To describe the composition dependence, 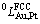 and
and  are necessary to be taken into account.
are necessary to be taken into account.
3.2 Optimization
The optimization was carried out by using thermodynamic calculation software (Thermo-Calc). Since the experimental data for the liquid phase are scarce, it is difficult to start with the assessment of the liquid phase. The thermodynamic parameters of the solid phases were thus firstly optimized. For the solid phase, the determined thermodynamic properties, including formational enthalpy [22,23] and the activity [21], were used together with our MG determined by diffusion couple technique [20]. The liquid phase was then investigated considering the phase diagram data. All the parameters were finally evaluated together to reproduce not only the experimental phase boundaries but also the thermodynamic data. All the evaluated parameters are listed in Table 1.
Table 1 Evaluated thermodynamic parameters for Au-Pt system

4 Results and discussion
The calculated Au-Pt binary phase diagram in this work is shown in Fig. 2, compared with the one by GROLIER and RAINER [18], including the experimental data [8,10,11,14,18,20]. The calculated results agree well with most of the experimental phase equilibria data. The results show that the present assessed MG deviates from the previous one, which shifts to the Au-rich side of the Au-Pt system. The critical point of the MG is at about 1200 ℃ at 56%Pt (molar fraction), in contrast to the previously assessed 1260 ℃ at 61%Pt [7]. In this work, the constraint of the third law of the thermodynamics is considered, and the two phases in equilibrium at 0 K are predicted to be pure Au and pure Pt.
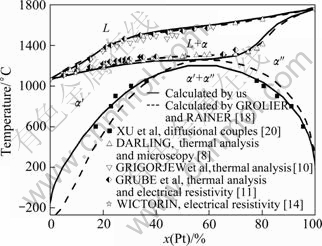
Fig. 2 Calculated phase diagrams of Au-Pt system in this work compared with results calculated by GROLIER et al, together with available experimental data
The calculated enthalpy of the formation of solid phase together with experimental data and calculated results previously is shown in Fig. 3 [18,21-23,26]. It is noted that the formation enthalpy is positive, which indicates a MG occurs in the solid phase. Moreover, some uncertainties can be observed between the experimental and calculated data. Taking the calculated data into account using activity and/or model, the calculated results are not fully considered. The present calculation puts emphasis on the consideration of these experimental data by KUBASCHEWSKI et al [22] and SCHALLER [23], and the results show a reasonable agreement with the experimental data. Moreover, the comparison between the experimental data and calculated results of the Au activity in solid phase is shown in Fig. 4 [18,21]. The present calculated results can properly reproduce the experimental data. However, the calculated results are slightly lower than the experimental data at 1200 ℃.

Fig. 3 Calculated formation enthalpy of FCC phase with reference to Au (FCC, 25 ℃) and Pt (FCC, 25 ℃), together with available experimental data and results calculated by GROLIER et al
In summary, the present thermodynamic parameters can reproduce the experimental phase boundaries and thermodynamic properties, especially obey the constraint of the third law of thermodynamics. This provides the basic information for the alloy design and future development of the Au-Pt multicomponent alloys, in particular, for understanding the high catalytic activity of Au-Pt-based alloy nanoparticles.
5 Conclusions
1) The Au-Pt system has been thermodynamically re-assessed using the Calphad approach by combining new experimental miscibility gap data, previous liquid data and thermodynamic properties, which well reproduces most experimental data and conforms to the third law of thermodynamics.
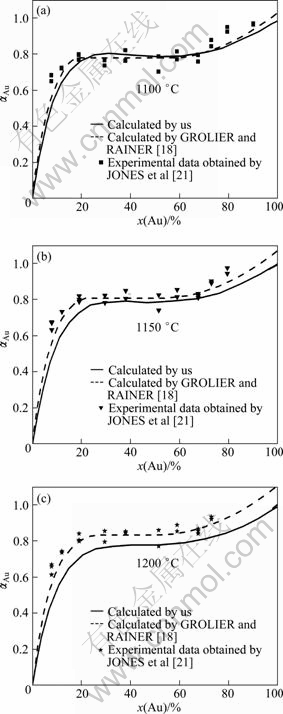
Fig. 4 Calculated activity of Au in FCC phase compared with results by GROLIER et al: (a) 1100 ℃; (b) 1150 ℃; (c) 1200 ℃ (FCC, given temperature)
2) According to the presently assessed results, the miscibility gap region slightly shifts to the Au-rich side of the Au-Pt system, and the critical point of the miscibility gap is about 1200 ℃ at Au-56%Pt, in contrast to 1260 ℃ at Au-61%Pt reported by previous assessment.
References
[1] HUANG Bing-xing, LIU Shi-chun, ZHANG Guo-qing, ZHANG Rui-hua. A new Au-Pt alloys electrode leader for gas sensor [J]. Precious Metals, 2000, 21(1): 12-17. (in Chinese)
[2] BRAEMER W. Biocompatibility of dental alloys [J]. Advanced Engineering Materials, 2001, 3(10): 753-761.
[3] WATAHA J C. Biocompatibility of dental casting alloys: A review [J]. Journal of Prosthetic Dentisty, 2000, 83(2): 223-234.
[4] SHIRAISHI T, TAKUMA Y, MIURA E, TANAKA Y, HISATSUNE K. Factors affecting the optical properties of Pd-free Au-Pt-based dental alloys [J]. Journal of Materials Science: Materials in Medicine, 2003, 14(12): 1021-1026.
[5] Jr COUTU R A, KLADITIS P E, LEEDY K D, CRANE R L. Selecting metal alloy electric contact materials for MEMS switches [J]. Journal of Micromechanics and Microengineering, 2004, 14(8): 1157-1164.
[6] M?LLER H, PISTORIUS P C. The electrochemistry of gold-platinum alloys [J]. Journal of Electroanalytical Chemistry, 2004, 570(2): 243-255.
[7] OKAMOTO H, MASSALSKI T B. The Au-Pt (glod-platinum) system [J]. Journal of Phase Equilibia, 1985, 6(1): 46-56.
[8] DARLING A S. Gold-platinum alloys: A critical review of their constitution and properties [J]. Platinum Metal Review, 1962, 6(3): 106-111.
[9] RUDOLF R, ZUPANCIC HARTNER T, KOSEC L, TODOROVIC A, KOSEC B, ANZEL I. Mechanical properties and microstructure characterization of Au-Pt dental alloy [J]. Metalurgia, 2008, 47(4): 317-323.
[10] GRIGORJEW A T. The gold-platinum alloys [J]. Zeitschift für Anorganische und Allgemeine Chemie, 1929, 178(1): 97-107.
[11] GRUBE G, SCHNEIDER A, ESCH U. Gold-platinum system [J]. Heraeus Festschrift, 1951, 8: 20-42.
[12] JOHANSSON C H, LINDE J O. Crystal structures, electrical resistance, thermal forces, heat conductivity, magnetic susceptibility, hardness and tempering phenomena in the system gold-platinum in relation to the phase diagram [J]. Annalen der Physik, 1930, 397(6): 762-792.
[13] STENZEL W, WEERTS J. X-ray examination of alloys of the gold-platinum system [J]. Siebert-Festschrift, 1931, 3(6): 300-308.
[14] WICTORIN C G. The separation of gold-platinum alloys [J]. Annalen der Physik, 1938, 425(6): 509-516.
[15] AGEL K. Separation curve and critical point of the system gold-platinum [J]. Zeitschift für Physikalische Chemie Neue Folge, 1960, 23(56): 415-425.
[16] TIEDEMA T J, BOUMAN J, BURGERS W G. Precipitation in gold-platinum alloys [J]. Acta Metallurgica, 1957, 5(6): 310-321.
[17] RAUB E, FALKENBURG G.. The system gold-platinum-rhodium and the binary system of its component [J]. Zeitschift Für Metallkunde, 1964, 55(4): 392-397.
[18] GROLIER V, RAINER S F. Experimental study of Au-Pt-Sn phase equilibria and thermodynamic assessment of the Au-Pt and Au-Pt-Sn systems [J]. Journal of Electronic Materials, 2008, 37(3): 264-278.
[19] VESNIN Y I, SHUBIN Y V. The equilibrium decomposition of Au-Pt solid solutions [J]. Journal of the Less Common Metals, 1988, 142: 213-219.
[20] XU X N, QIN G W, REN Y P, SHEN B, PEI W L. Experimental study of the miscibility gap and calculation of the spinodal curves of the Au-Pt system [J]. Scripta Materialia, 2009, 61: 859-862.
[21] JONES R W, STAFFORD F E, WHITMORE D H. Mass spectrometric study of the thermodynamic properties of solid Au-Pt alloys [J]. Metallurgical Transactions, 1970, 1(2): 403-413.
[22] KUBASCHEWSKI O, ALCOCK C B, SPENCER P J. Materials Thermochemistry [M]. Oxford: Pergamon, 1993: 212-215.
[23] SCHALLER H J. A thermodynamic analysis of the Pt-Au miscibility gap [J]. Zeitschift für Metallkunde, 1979, 70(6): 354-358.
[24] RUDNITSKII A A. Thermoelectric properties of the noble metals and their alloys [M]. Moscow: Atomic Enegy Commission, 1956: 197-202.
[25] JOHANSSON C H, HAGSTEN O. Proof of hysteresis between and dissociation and reformation of the homogeneous metal phase [J]. Annalen der Physik, 1937, 420(6): 520-527.
[26] De BOER F R, BOOM R, MATTENS W C M, MIEDEMA A R, NIESSEN A K. Cohesion in metals: Transition metal alloys [M]. Amsterdam: NothHolland, 1988: 426-432.
Au-Pt二元合金的热力学评估
徐晓宁1,任玉平1,李长发2,李 松1,秦高梧1
1. 东北大学 材料各向异性与织构教育部重点实验室,沈阳 110819;
2. 天津理工大学 材料科学与工程学院,天津 300384
摘 要:基于最近实验测得的Au-Pt二元体系相平衡数据,利用Calphad方法重新评估Au-Pt二元体系的热力学参数。采用亚正规溶体模型Redlich-Kister等式描述液相和面心立方相的Gibbs自由能。考虑热力学第三定律的限定,以再现相平衡数据和固相热力学性质,包括活度和混合焓,优化Au-Pt二元系统热力学参数。优化结果表明: Au-Pt合金系统的溶解度间隙边界向富Au侧偏移,其顶点位置在1200 ℃,Au-56%Pt。
关键词:Au-Pt体系;相图;热力学评估;Calphad法
(Edited by FANG Jing-hua)
Foundation item: Project (50871028) supported by the National Natural Science Foundation of China; Projects (N100702001, N090502002) supported by the Fundamental Research Funds for the Central Universities, China; Project (NCET-09-0272) supported by the Program for New Century Excellent Talents in University of Ministry of Education, China; Project (200803) supported by Northeastern University Research Foundation for Doctor Candidates, China
Corresponding author: QIN Gao-wu; Tel: +86-24-83683772; Fax: +86-24-83686455; E-mail: qingw@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(11)61337-X