高分散Li4Ti5O12纳米晶的制备及电化学性能
来源期刊:中国有色金属学报(英文版)2012年第3期
论文作者:王瑾 刘晓敏 杨晖
文章页码:613 - 620
关键词:锂离子电池;Li4Ti5O12;纳米晶;表面活性剂;溶胶?凝胶法
Key words:lithium-ion battery; lithium titanate; nanocrystals; surfactant; sol-gel method
摘 要:
以月桂酸为分散剂,采用溶胶-凝胶法合成颗粒尺寸为120~250 nm的高分散Li4Ti5O12纳米晶。通过研究表面活性剂月桂酸的含量优化制备工艺,制备出电化学性能最佳的样品。采用XRD、FESEM、TEM、激光粒度分析仪、交流阻抗以及恒流充放电测试,对材料的物理和电化学性能进行表征。在800 ℃下热处理10 h后的高分散性Li4Ti5O12纳米晶显示出优异的电化学性能,在1C倍率下,首次放电容量为163.3 mA?h/g,50次放电循环后,放电容量无明显衰减。研究表明,高分散性Li4Ti5O12纳米晶可以缩短锂离子的扩散路径,改善样品的电化学动力过程,有效地提高其高倍率性能。
Abstract:
A sol-gel method using lauric acid as surfactant was used to synthesize Li4Ti5O12 nanocrystals with an ultra-fine particle size distribution between 120 and 250 nm. In order to obtain the electrode materials with the best electrochemical performance, the content of lauric acid during Li4Ti5O12 synthesis was systematically studied. The physical and electrochemical properties of the synthesized samples were characterized by X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), laser particle size analysis, alternating current impedance (AC) and galvanostatic charge-discharge experiments. The highly dispersed Li4Ti5O12 nanocrystals obtained at 800 ℃ for 10 h can deliver a specific capacity of 163.3 mA?h/g at 1C rate without obvious capacity fade up to 50 cycles. The results suggest that well dispersed Li4Ti5O12 nanocrystals shorten the Li-ion diffusion length and enhance the electrochemical kinetics of the samples, which are very crucial to high rate capability.

![]()

Trans. Nonferrous Met. Soc. China 22(2012) 613-620
WANG Jin1, 2, LIU Xiao-min1, YANG Hui2
1. College of Materials Science and Engineering, Nanjing University of Technology, Nanjing 210009, China;
2. National Engineering Research Center for Nanotechnology, Shanghai 200241, China
Received 23 March 2011; accepted 23 November 2011
Abstract: A sol-gel method using lauric acid as surfactant was used to synthesize Li4Ti5O12 nanocrystals with an ultra-fine particle size distribution between 120 and 250 nm. In order to obtain the electrode materials with the best electrochemical performance, the content of lauric acid during Li4Ti5O12 synthesis was systematically studied. The physical and electrochemical properties of the synthesized samples were characterized by X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), laser particle size analysis, alternating current impedance (AC) and galvanostatic charge-discharge experiments. The highly dispersed Li4Ti5O12 nanocrystals obtained at 800 °C for 10 h can deliver a specific capacity of 163.3 mA·h/g at 1C rate without obvious capacity fade up to 50 cycles. The results suggest that well dispersed Li4Ti5O12 nanocrystals shorten the Li-ion diffusion length and enhance the electrochemical kinetics of the samples, which are very crucial to high rate capability.
Key words: lithium-ion battery; lithium titanate; nanocrystals; surfactant; sol-gel method
1 Introduction
At present, state-of-the-art Li-ion batteries have been considered an attractive power source for a wide variety of applications due to their high power and energy densities [1-6]. Their higher power density than present commercial Li-ion batteries can support some industrial applications, such as in electric vehicles and hybrid electric vehicles. Nano-sized electrode materials have been demonstrated to facilitate electrochemical reactions and improve the power density of Li-ion batteries because their shorter Li-ion transport length and higher contact area between the electrode and electrolyte enable higher power [7-9]. In addition to the particle size, good dispersion of the particles may also be crucial to the high rate performance of Li-ion batteries. As a promising anode material for Li-ion batteries, spinel Li4Ti5O12 has attracted special attention due to its extremely small structural change during Li insertion/extraction, its high reversible capacity (175 mA·h/g) and its flat discharge platform at about 1.55 V versus Li+/Li [10-13]. This high voltage above the reduction potential of most organic electrolytes can restrain the passive films from the reduction of electrolytes and sufficiently avoid the formation of metallic lithium [14].
Li4Ti5O12 powders are usually synthesized by conventional solid-state reaction of lithium and titanium sources [10-13, 15]. Solid-state methods have some disadvantages, such as high calcination temperature, large particle size, impurity phases and lack of stoichiometry control. Compared with solid-state reaction, sol-gel route can obviously overcome these disadvantages. KIM et al [16] have synthesized Li4Ti5O12 with high electrochemical performance using the polyol process in non-aqueous media. JIANG et al [17] have prepared highly dispersed Li4Ti5O12 nano-particles by the P123 surfactant-assisted sol-gel process, exhibiting very good rate capability. A lot of research has proven that highly dispersed Li4Ti5O12 nanocrystalline can enhance high rate performance and rate capability [17, 18]. Recently, CHOI et al [19] reported improving the electrochemical performance of LiFePO4 electrodes by controlling the particle morphology with a nonaqueous sol-gel process using lauric acid as a surfactant, but the dispersion mechanism of the lauric acid was not reported. Such a surfactant based sol-gel route offers a novel and simple method to synthesize Li4Ti5O12 nanocrystalline. In the present study, we report a sol-gel route using lauric acid as a surfactant to prepare highly dispersed Li4Ti5O12 nano-particles and demonstrate the effect of grain size and dispersion of Li4Ti5O12 nanocrystalline on the high rate capability.
2 Experimental
2.1 Synthesis of Li4Ti5O12
Nanocrystalline Li4Ti5O12 was synthesized using CH3COOLi·2H2O (AR), tetrabutyl titanate [Ti(OC4H9)4], (CP) and lauric acid (CP). Stoichiometric amounts of CH3COOLi·2H2O and Ti(OC4H9)4 were respectively dissolved in alcohol to obtain homogeneous solutions A and B. Solution A was added slowly to solution B with a mole ratio of Li to Ti equal to 1. The mixed solution was magnetically stirred at room temperature to get a transparent yellow solution. To the formed solution, an ethanol solution of lauric acid was added under constant stirring at room temperature, with the molar ratio of lauric acid to titanium, LTR, 0.1, 0.16, 0.2 and 0.4. The mixed solution was then stirred at room temperature for about 20 h to form a white sticky gel. After aging in air for several hours, the resulting gel was dried at 100 °C for 10 h. The gel precursors were preheated at 500 °C in air for 6 h and then calcined at 800 °C for 10 h to obtain Li4Ti5O12 powders. Li4Ti5O12 was also prepared without using lauric acid by a similar route for comparison. The Li4Ti5O12 sample prepared without lauric acid was labeled as pure LTO. In addition, the Li4Ti5O12 samples prepared with molar ratio of lauric acid to titanium, LTR=0.1, 0.16, 0.2 and 0.4, were marked as LTO-1, LTO-2, LTO-3 and LTO-4.
2.2 Characterization of Li4Ti5O12
The crystal structure of the synthesized powders was examined by X-ray diffraction analysis (XRD, Model X’TRAX) using nickel filtered Cu Kα radiation (λ=0.15406 nm) over the 2θ range from 10° to 80°. The particle morphology of the powders was observed using a JSM-6700F field emission scanning electron microscope (FESEM) and a Joel JEM-1010 transmission electron microscope (TEM). The particle size and distribution of the powders were measured with a laser particle size analyzer (Malvern, Mastersizer 2000).
2.3 Electrochemical characterization
Electrochemical properties of the samples were measured with assembled swagelok cells. Li metal was used as a counter and reference electrodes, the electrolyte was 1 mol/L LiPF6 in ethylene carbonate and diethyl carbonate (EC-DMC 1:1, V/V), and a Celgard3501 polypropylene micro-porous film was used as the separator. The Li4Ti5O12 electrode was prepared by mixing 85% Li4Ti5O12 active material, 10% carbon black (Super P) and 5% polyvynilidene ?uoride (PVDF) binder dispersed in enough N-methyl-2-pyrrolidine (NMP). Then, the viscous slurry was cast on a current collector of a copper foil by a blade. After drying overnight under vacuum at 100 °C to remove the solvent, the electrode was punched to a disk shape with a diameter of 16 mm for the half-cell test. The cell was assembled in a dry glove box filled with high purity argon gas. The galvanostatic discharge-charge tests were carried out using an Arbin Instument in the voltage range of 1.0-3.0 V versus Li+/Li. Electrochemical impedance spectroscopy was performed by an electrochemical workstation (Shanghai Chenhua Instrument Co., Ltd., China) in the frequency ranging from 0.1 Hz to 1 MHz.
3 Results and discussion
3.1 Structural analysis
Figure 1 displays XRD patterns of the samples prepared without lauric acid or with different contents of lauric acid. All the XRD patterns for the samples prepared without or with lauric acid exhibit the spinel Li4Ti5O12 phase without any impurity, which are completely consistent with the XRD pattern of spinel Li4Ti5O12 listed in JCPDS file No. 26—1198.

Fig. 1 X-ray diffraction patterns of samples prepared without lauric acid or with different contents of lauric acid
The crystallite size of Li4Ti5O12 was calculated from the Scherrer formula D=βλ/(Bcosθ), where B is the full-width-at-half-maximum (FWHM) of the diffraction peaks; λ is the X-ray wavelength (0.15418 nm); β is a constant (0.9); θ is the reflection angle of the peaks. Peaks at (111) reflection are taken to evaluate the crystallite size of Li4Ti5O12 crystallines. The mean crystallite sizes of Li4Ti5O12 particles are found to be 69.3, 67.8, 64.3, 62.2 and 65.2 nm, for pure LTO, LTO-1, LTO-2, LTO-3 and LTO-4 samples, respectively. The results indicate that lauric acid acts as a surfactant and also plays an important role as a chelating agent. Appropriate lauric acid is favorable to improve the dispersion of the gel and obtain Li4Ti5O12 powders with a small particle size. The cubic lattice parameters of Li4Ti5O12 crystallites are calculated to be 0.8365 and 0.8364 nm, for pure LTO and LTO-3 samples, respectively, in good agreement with those obtained by OHZUKU et al [10].
3.2 Morphology of Li4Ti5O12 samples
In order to investigate the effect of the quantity of lauric acid on the particle size and the morphology, the field emission scanning electron micrographs of the synthesized LTO, LTO-1, LTO-2, LTO-3 and LTO-4 powders with low and high magnification are shown in Fig. 2. Generally, the crystallite size of Li4Ti5O12 is about 150 nm in all these samples. These fine Li4Ti5O12 powders can be attributed to the sol-gel route in which an atomic level mixing of elements is achieved. From Figs. 2(a) and (b) it can be seen that the pure LTO sample appears as heavily aggregated micron-sized particles of 1-2 μm in diameter. We assume that these densely agglomerated particles can make the Li+ insertion/extraction in individual Li4Ti5O12 grains inhomogeneous. The Li4Ti5O12 grains inside the densely packed particles might be inactive especially during cycle at high current densities due to the increased Li+ diffusion distance. These results manifest that the aggregation is a significant problem related to the conventional sol-gel method although fine crystals can also be prepared.
However, lauric acid surfactant can effectively reduce the aggregation of Li4Ti5O12 nano-sized particles prepared by the sol-gel route. As a consequence, all the samples prepared with lauric acid exhibit well dispersed nanocrystallines without any agglomeration. For LTO-1 (Figs. 2 (c) and (d)) and LTO-2 (Figs. 2 (e) and (f)) samples, the average particle sizes of Li4Ti5O12 powders are 300-450 nm and they have no significant differences. When the molar ratio of lauric acid to titanium increases to 0.2 (sample LTO-3), it can be observed that the average particle size decreases to 300-400 nm, as seen in Figs. 2 (g) and (h). Especially, the small difference of the particle size can be observed in the FE-SEM micrographs with high magnification. However, a further increase in LTR to 0.4 (sample LTO-4) causes the average particle size to increase to 300-450 nm, as shown in Figs. 2(i) and (j). The gel time and crystalline sizes of these samples evaluated by the XRD patterns are listed in Table 1. The time of gel formation gradually increases with the increase of the quantity of lauric acid. In the gelation, lauric acid as an organic anionic surfactant can absorb on the surface of colloidal particles with its long zigzag chain, thus inhibiting colloid grain coalescence, leading to well-dispersed Li4Ti5O12 grains. The schematic diagram of dispersion mechanism for lauric acid is shown in Fig. 3. In the complexation, lauric acid can effectively delay the hydrolysis reaction and significantly improve the uniformity of the colloidal network, thus promoting a narrow size distribution of Li4Ti5O12 powders. Lauric acid not only acts as the dispersant and the chelating agent, but also provides the local heat for the formation of compounds during the decomposition, thus effectively decreasing the formation temperature and time of pure Li4Ti5O12. Unfortunately, excessive lauric acid generates superfluous heat, thus accelerating the growth of the crystallite. These results show that the appropriate quantity of the surfactant is important for the synthesis of Li4Ti5O12 powders with effectively controlled particle size, thus significantly affecting the electrochemical properties of the Li4Ti5O12 powders. As the LTO-3 sample exhibits the best dispersivity and the finest particle size, the appropriate content of lauric acid is chosen as molar ratio of lauric acid to titanium, LTR=0.2.
Table 1 Gel time and crystalline sizes of Li4Ti5O12 powders

The particle size distributions of the Li4Ti5O12 powders for the pure LTO and LTO-3 samples determined by the laser particle size analyzer are depicted in Fig. 4. The particle size distributions clearly show the large difference between the average particle sizes in both samples. As seen in Figs. 2(a) and (b) and Figs. 2(g) and (h), the average particle sizes of the Li4Ti5O12 powders for the pure LTO and LTO-3 samples are 439 nm and 188 nm, respectively. The specific results are listed in Table 2. Therefore, these results show that the lauric acid surfactant-assisted sol-gel approach is suitable for preparing well-dispersed Li4Ti5O12 nanocrystals with high crystallinity.
In order to further explore the difference in the particle size and dispersivity of both Li4Ti5O12 samples, the microstructures are studied by TEM. Figure 5 presents TEM images of the pure LTO and LTO-3 samples. The sample prepared without surfactant (pure LTO) manifests dense micron-sized particles due to heavy aggregation. The heavy aggregation of the sample LTO-3 containing well-dispersed grains cannot be fully penetrated by the electron beam, and thus shows dark and dense micron-sized particles. In contrast, it exhibits a diameter of about 200 nm, as evaluated by the XRD results. This indicates that highly dispersed Li4Ti5O12 nanocrystals have been successfully fabricated by the lauric acid surfactant-assisted sol-gel route.
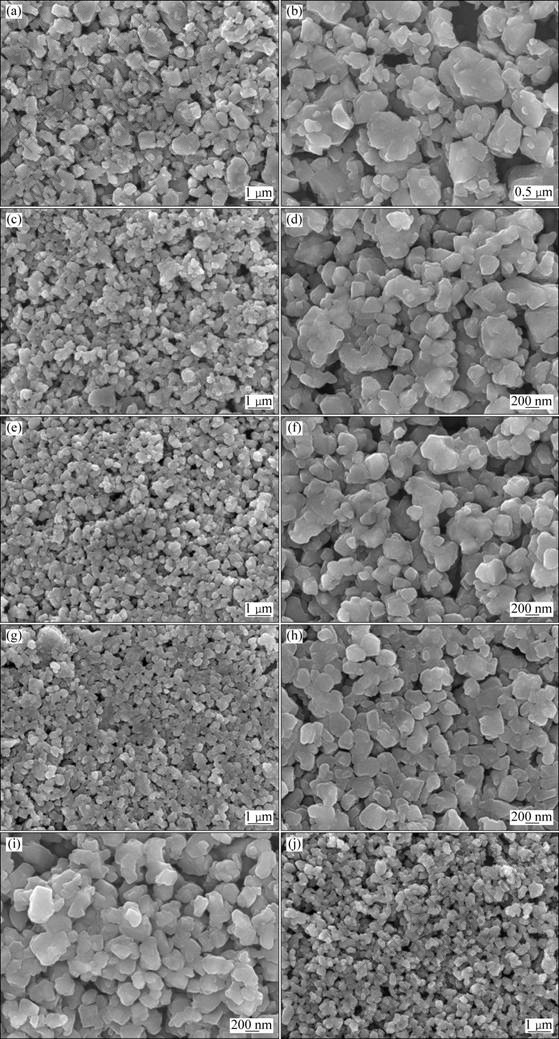
Fig. 2 FE-SEM images of pure LTO (a, b), LTO-1 (c, d), LTO-2 (e, f), LTO-3 (g, h), and LTO-4 (i, j) samples
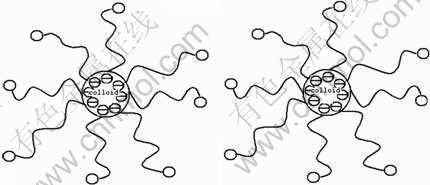
Fig. 3 Schematic diagram of dispersion mechanism for lauric acid
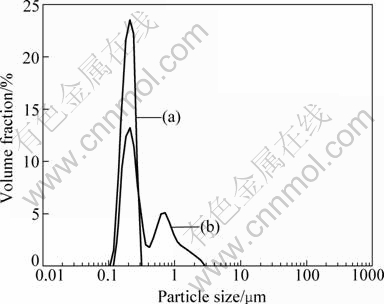
Fig. 4 Particle size distribution of LTO-3 (a) and pure LTO (b) samples
Table 2 Particle size distribution of Li4Ti5O12 powders for LTO-3 and pure LTO samples

3.3 Electrochemical analysis
The effect of the quantity of lauric acid on the electrochemical performance of Li4Ti5O12 samples is clarified by galvanostatic discharge-charge measurements between 1.0 V and 3.0 V versus Li+/Li at 0.5C rate. Figure 6 shows the discharge-charge curves of these samples. As shown in Fig. 6, the discharge capacities reach 152, 148.7, 174.7 and 158 mA·h/g, respectively, for the LTO-1, LTO-2, LTO-3 and LTO-4 samples. The sample LTO-3 exhibits the best electrochemical performance with a specific capacity of 174.7 mA·h/g and 97.9 % coulombic efficiency, at 0.5C rate. According to the FE-SEM patterns (Fig. 2(g) and (h)), the sample LTO-3 manifests the smallest particles, and also delivers the best discharge capacity. Thus, it demonstrates that the particle size significantly affects electrochemical properties. HAO et al [20] reported that the particle size, shape and homogeneity of electrode materials had a definite effect on the electrochemical properties, and among them, particle size was the main factor. JIANG et al [17] reported that Li4Ti5O12 nano-powders exhibited good Li storage capacity and cycle stability even at high C-rates. As the nano-sized particle can reduce the ion-diffusion pathway, thus favoring Li-ion mobility, the capacity can be improved significantly. According to the results of the morphology and the electrochemical performance, the suitable molar ratio of lauric acid to titanium is determined to be 0.2.

Fig. 5 TEM images of LTO-3 (a) and pure LTO (b) samples

Fig. 6 Discharge-charge curves of Li4Ti5O12 electrodes prepared from LTO-1 (a), LTO-2 (b), LTO-3 (c) and LTO-4 (d) samples at 0.5C rate
According to the FE-SEM patterns (Figs. 2(a) and (b) and Figs. 2(g) and (h)), Li4Ti5O12 synthesized using lauric acid surfactant (LTO-3) exhibits well dispersed nano-sized particles with almost no aggregation, whereas heavily aggregated micron-sized particles (pure LTO) are prepared without surfactant. In order to investigate the effect of the morphologies of Li4Ti5O12 powders on the electrochemical properties, the discharge-charge tests of these two samples prepared with and without surfactant were carried out under different constant current densities. Figure 7 shows the discharge curves of these two samples at different rates. The Li4Ti5O12 sample prepared with surfactant delivers a discharge capacity of 174.7 mA·h/g at 0.5C rate. In contrast, the discharge capacity of the Li4Ti5O12 electrode prepared without surfactant is only 146.9 mA·h/g. However, with increasing the discharge-charge current rate, the difference between the discharge capacities of these two samples becomes evident. The discharge capacity of the Li4Ti5O12 electrode with surfactant is 132.3 mA·h/g at 2C rate. However, the discharge capacity of Li4Ti5O12 prepared without surfactant drops rapidly to 91.5 mA·h/g at 2C rate. It is found that the well-dispersed nano-sized Li4Ti5O12 sample exhibits a higher discharge capacity than the highly aggregated sample. In addition, the capacity-cycle profile of Li4Ti5O12 prepared without surfactant shows a large fluctuation in capacity, which is not as smooth as that of the well-dispersed Li4Ti5O12 sample, as shown in Fig. 8. The excellent cycling behavior of Li4Ti5O12 prepared with surfactant may be attributed to high crystallinity and well-dispersed nano-sized particles, as shown in Figs. 2(g) and (h), which results in a smooth capacity-cycle profile with the average capacity fading of 0.2% per cycle within the first 50 cycles at 1C rate. JIANG et al [17] reported that this capacity fluctuation suggested that the severe aggregation, which makes lithium ion insertion/ extraction in individual Li4Ti5O12 grains inhomogeneous in different cycles. We believe that a large portion of Li4Ti5O12 grains in the aggregated particles cannot be fully utilized and become inactive due to a longer diffusion distance for the lithium ion and insufficient Li-ion diffusion at high rates, which inevitably decreases the discharge capacity. Li-ion insertion/extraction mainly takes place at the outside of the large particles and individual Li-ion insertion/extraction in the aggregated particles at different cycles leads to the capacity fluctuation in the capacity-cycle profile. It should be noted that the well-dispersed Li4Ti5O12 nano-particles not only can increase the contact area between the active materials and the carbon black and then improve the electrochemical performance, but also are extremely important to obtain a homogenous mixture of Li4Ti5O12 and carbon black. Well-dispersed Li4Ti5O12 nano- particles lead to a uniform mixing with carbon black. Therefore, the carbon black homogenously mixed with the active materials promotes partial Li insertion and extraction. As stated above, it also demonstrates that good dispersion can significantly improve the electrochemical kinetics of Li4Ti5O12 samples by increasing the contact area, reducing the particle size and favoring lithium ion and electron transport, thus enhancing high rate performance.

Fig. 7 Discharge curves at different C-rates for Li4Ti5O12 electrodes prepared from pure LTO (a) and LTO-3 (b) samples
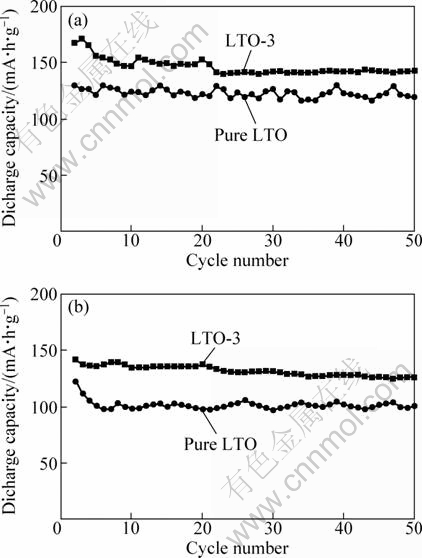
Fig. 8 Cycle performance of Li4Ti5O12 electrodes prepared from LTO-3 and pure LTO samples at 0.5C (a) and 1C (b) rates
Figure 9 shows typical impedance spectra of Li4Ti5O12 electrodes prepared from the pure LTO and LTO-3 samples at 0% depth of discharge (DOD) (3 V vs Li+/Li) and 40% DOD (1.55 V vs Li+/Li)), respectively. The impedance plots are composed of a depressed semicircle in the high frequency range and a spike in the low frequency range. The high-frequency semicircle is associated with the charge transfer process, and the spike in the low frequency range can be attributed to the Warburg impedance of long-range Li-ion diffusion through the Li4Ti5O12 electrode [21]. It can be obviously seen that the resistance of the Li4Ti5O12 electrode prepared with surfactant is lower than that of the Li4Ti5O12 electrode prepared with the pure LTO sample, which demonstrates that good dispersion can significantly improve the electrochemical kinetics of Li4Ti5O12 samples and decrease their resistance. On the other hand, the resistance of the samples at 0% DOD (3 V vs Li+/Li) is lower than the resistance at 40% DOD (1.55 V vs Li+/Li). This suggests that the resistance increases with the decrease of the cell voltage. In the discharge process, with the increase of depth of discharge, more and more lithium ions are inserted into the electrode materials, so that the coulomb repulsion of the lithium ions is reinforced, which hinders Li-ion mobility, causing the increase of charge transfer resistance and thus the total resistance of the Li4Ti5O12 electrode. Therefore, the resistance increases with the increase of DOD. From all of the above results, we conclude that well-dispersed Li4Ti5O12 nanocrystals can shorten Li-ion diffusion pathway, favoring Li-ion mobility and enhancing high rate performance.
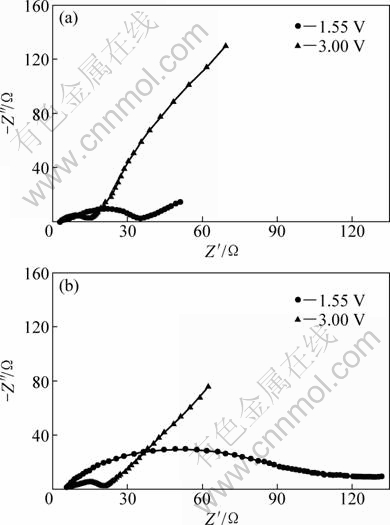
Fig. 9 Electrochemical impedance spectra of Li4Ti5O12 electrodes at 0% DOD (3.0 V vs Li+/Li) and 40% DOD (1.55 V vs Li+/Li) prepared from LTO-3 (a) and pure LTO (b) samples
4 Conclusions
Highly dispersed, nanocrystalline Li4Ti5O12 was successfully synthesized by the surfactant assisted sol-gel method using lauric acid as the surfactant. The Li4Ti5O12 sample prepared with the surfactant shows a first discharge capacity of 163.3 mA·h/g at 1C rate, which is obviously better than the severely aggregated Li4Ti5O12 particles prepared without surfactant. The results suggest that well dispersed Li4Ti5O12 nanocrystalline shortens the Li-ion diffusion length and enhances the electrochemical kinetics of the samples, which are very crucial to high rate capability. The current sol-gel approach to synthesize highly dispersed nanocrystals can be proven to also be appropriate for preparing other electrode materials with good electrochemical performance.
References
[1] AMATUCCI G G, BADWAY F, PASQUIER A D, ZHENG T. An asymmetric hydrid nonaqueous energy storage cell [J]. J Electrochem Soc A, 2001, 148: A930-A939.
[2] ARMAND M, TARASCON J M. Building better batteries [J]. Nature, 2008, 451: 652-657.
[3] TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries [J]. Nature, 2001, 414: 359-367.
[4] JANSEN A N, KAHAIAN A J, KEPLER K D, NELSON P A, AMINE K, DEES D W, VISSERS D R, THACKERAY M M. Development of a high-power lithium-ion battery [J]. J Power Sources, 1999, 81-82: 902-905.
[5] TAKEI K, ISHIHARA K, KUMAI K, IWAHORI T, MIYAKE K, NAKATSU T, TERADA N, ARAI N. Performance of large-scale secondary lithium batteries for electric vehicles and home-use load-leveling systems [J]. J Power Sources, 2003, 119-121: 887-892.
[6] NAZAR L F, GOWARD G, LEROUX F, DUNCAN M, HUANG H, KERR T, GAUBICHER J. Nanostructured materials for energy storage [J]. Int J Inorg Mater, 2001, 3: 191-200.
[7] FICHTNER M, ENGEL J, FUHR O, KIRCHER O, RUBNER O. Nanocrystalline aluminium hydrides for hydrogen storage [J]. Mater Sci Eng B, 2004, 108: 42-47.
[8] JIANG C H, HOSONO E, ICHIHARA M, HONMA I, ZHOU H S. Synthesis of nanocrystalline Li4Ti5O12 by chemical lithiation of anatase nanosrystals and postannealing [J]. J Electrochem Soc A, 2008, 155(8): A553-A556.
[9] SIDES C R, MARTIN C R. Nanostructured electrodes and the low-temperature performance of Li-ion batteries [J]. Adv Mater, 2005, 17(1): 125-128.
[10] OHZUKU T, UEDA A, YAMAMOTO N. Zero-strain insertion material of Li[Li1/3Ti5/3]O4 for rechargeable lithium cells [J]. J Electrochem Soc, 1995, 142(5): 1431-1435.
[11] MUKAI K, ARIYOSHI K, OHZUKU T. Comparative study of Li[CrTi]O4, Li[Li1/3Ti5/3]O4 and Li1/2Fe1/2[Li1/2Fe1/2Ti]O4 in non-aqueous lithium cells [J]. J Power Sources, 2005, 146: 213-216.
[12] ZAGHIB K, SIMONEAU M, ARMAND M, GAUTHIER M. Electrochemical study of Li4Ti5O12 as negative electrode for Li-ion polymer rechargeable batteries [J]. J Power Sources, 1999, 81-82: 300-305.
[13] ARIYOSHI K, YAMATO R, OHZUKU T. Zero-strain insertion mechanism of Li[Li1/3Ti5/3]O4 for advanced lithium-ion (shuttlecock) batteries [J]. Electrochim. Acta, 2005, 51: 1125-1129.
[14] PROSINI P P, MANCINI R, PETRUCCI L, CONTINI V, VILLANO P. Li4Ti5O12 as anode in all-solid-state, plastic, lithium-ion batteries for low-power applications [J]. Solid State Ionics, 2001, 144: 185-192.
[15] HUANG C K, SAKAMOTO J S, WOLFENSTINE J, SURAMPUDI S. The limits of low-temperature performance of Li-ion cells [J]. J Electrochem Soc, 2000, 147(8): 2893-2896.
[16] KIM D H, AHN Y S, KIM J. Polyol-mediated synthesis of Li4Ti5O12 nanoparticle and its electrochemical properties [J]. Electrochem Commun, 2005, 7: 1340-1344.
[17] JIANG C H, ICHIHARA M, HONMA I, ZHOU H S. Effect of particle dispersion on high rate performance of nano-sized Li4Ti5O12 anode [J]. Electrochim Acta, 2007, 52: 6470-6475.
[18] NAKAHARA K, NAKAJIMA R, MATSUSHIMA T, MAJIMA H. Preparation of particulate Li4Ti5O12 having excellent characteristics as an electrode active material for power storage cells [J]. J Power Sources, 2003, 117: 131-136.
[19] CHOI D, KUMTA P N. Surfactant based sol-gel approach to nanostructured LiFePO4 for high rate Li-ion batteries [J]. J Power Sources, 2007, 163: 1064-1069.
[20] HAO Y J, LAI Q Y, LU J Z, WANG H L, CHEN Y D, JI X Y. Sythesis and characterization of spinel Li4Ti5O12 anode material by oxalic acid-assisted sol-gel method [J]. J Power Sources, 2006, 158: 1358-1364.
[21] HUANG J J, JIANG Z Y. The preparation and characterization of Li4Ti5O12/carbon nano-tubes for lithium ion battery [J]. Electrochim Acta, 2008, 53: 7756-7759.
王 瑾1, 2,刘晓敏1,杨 晖2
1. 南京工业大学 材料科学与工程学院,南京 210009;
2. 纳米技术及应用国家工程研究中心,上海 200241
摘 要:以月桂酸为分散剂,采用溶胶-凝胶法合成颗粒尺寸为120~250 nm的高分散Li4Ti5O12纳米晶。通过研究表面活性剂月桂酸的含量优化制备工艺,制备出电化学性能最佳的样品。采用XRD、FESEM、TEM、激光粒度分析仪、交流阻抗以及恒流充放电测试,对材料的物理和电化学性能进行表征。在800 °C下热处理10 h后的高分散性Li4Ti5O12纳米晶显示出优异的电化学性能,在1C倍率下,首次放电容量为163.3 mA·h/g,50次放电循环后,放电容量无明显衰减。研究表明,高分散性Li4Ti5O12纳米晶可以缩短锂离子的扩散路径,改善样品的电化学动力过程,有效地提高其高倍率性能。
关键词:锂离子电池;Li4Ti5O12;纳米晶;表面活性剂;溶胶-凝胶法
(Edited by YANG Hua)
Foundation item: Project (2007CB2097050) supported by the National Basic Research Program of China; Project (20803035) supported by the National Natural Science Foundation of China; Project supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), China
Corresponding author: YANG Hui; Tel: +86-25-83587238; E-mail: yanghui@njut.edu.cn; LIU Xiao-min; E-mail: lxm3799@yahoo.com
DOI: 10.1016/S1003-6326(11)61222-3

