
Effects of thickness of Al thermal spray coating for STS 304
Min-Su HAN1, Yong-Bin WOO1, Seok-Cheol KO2, Young-Jae JEONG3,
Seok-Ki JANG1, Seong-Jong KIM1
1. Division of Marine Engineering, Mokpo Maritime University, Mokpo City, Jeonnam, 530-729, Korea;
2. Chungnam Regional Innovation Agency, 506 Jiksan-eup, Sameun-ri, Cheonan City, Chungnam, 330-816, Korea;
3. Jeonnam Techno Park, Suncheon City, Jeonnam, 540-856, Korea
Received 18 June 2008; accepted 10 March 2009
Abstract: Thermal spray coating presents a sacrificial-anode cathodic protection method since differences between the potentials of materials are employed. If a base metal is exposed to seawater, due to defect in the thermal spray coating, the metal is protected by the sacrificial anodic characteristics of the coating layer because micro-cells formed by the base metal and the thermal spray coating act as cathodes and anodes, respectively. The coating thickness has little effect on the surface morphology of the coating. However, the electrochemical properties with the increasing of coating thickness present good results.
Key words: aluminum; thermal spray coating; STS 304
1 Introduction
In marine environments, extreme corrosion occurred in all types of stainless steel, including STS 304, which can lead to serious problems if it occurs intensively in a vessel that does not have a stern tube cooling system on the surface of the propeller shaft in the stern tube, or in the clearance between the stern tubes and the surrounding wood[1-2]. Comprehensive investigations have been performed to determine an appropriate corrosion countermeasure[3-7]. Attempts have been made to protect stainless steel shaft systems from corrosion by installing grounding plates on the hull or using anticorrosive paint. However, the problem still exists and a definitive alternative technology has not yet been established. Pitted and cracked shafts must be repaired in dry docks by grinding and welding. Differences of opinion often exist between an inspector certifying the repairs and the companies performing the repairs due to ambiguities in the regulations. The thermal spraying coating materials for the corrosion protection are Al, Zn and Zn-15% Al. In the previous investigation, the Al thermal spraying coating material presented the best corrosion protection characteristics[3-7]. Therefore, in this work the effects of Al coating thickness were evaluated with the point of microstructure and electro- chemical property.
2 Experimental
The aluminum thermal spray wire (1.6 mm) of above 99.7% purity was used for STS 304 base metal. The thermal spray equipment (Arcspray Equipment Type OSU-Haussler 300A antiCOR) was used. The electrochemical experiment was executed in natural seawater and the specimen was made in 2 cm×2 cm sizes and put in the manufactured holder. Corrosion potential measurements were recorded over a period of 86 400 s at room temperature using an electro-chemical apparatus consisting of a Pt coil as the counter-electrode and an Ag/AgCl-saturated KCl as the reference electrode. Anodic polarization experiments were executed from an open circuit potential(OCP) to a voltage ranging from 0 to 5.0 V with a scan rate of 2 mV/s. Cathodic polarization experiments were performed from an OCP to -2.0 V. Potentiostatic experiments for 1 200 s at an applied various potential were also performed. The current density after 1 200 s was compared with various applied potential conditions. The coating layers were analyzed with SEM and EDX.
3 Results and discussion
Fig.1 shows the surface and cross-section morphologies with different thermal spray coating thickness. Plate shape was observed at surface microstructure of zinc coating material. The reason is that solidification occurs while molten metal particle of high velocity clashes base metal surface and crushes it[8]. The reason why thermal spray layer forms is due to the specific characteristics of plate accumulation structure and formation of large porosity because of incomplete plate accumulation[9-10], and melted particle by flame of high temperature at thermal spray spreads platy or radial shape after clashing base metal with high velocity. After that, small particle is separated in the thin and unstable end part, and porosity of middle size is formed, when the plate is made while melting particles strike solidified particle in the end of splat[10]. In addition, while plate was made from the process that gas isolated in melting particle strikes base metal in the flame of high temperature, small porosity is formed inside when gas evaporates. Because of existing many groove, the corrosion resistance is decreased due to the porous coating layer. The coating layer thickness affects largely the corrosion resistance.
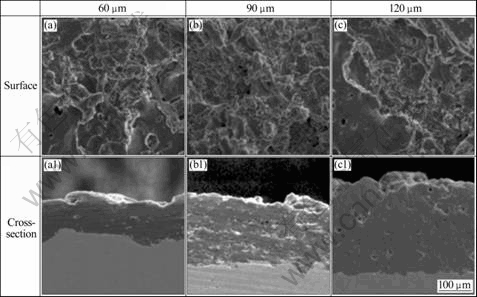
Fig.1 Surface and cross-section morphologies of Al thermal spray coatings with different thickness
Fig.2 shows the EDX composition distribution of Al coatings with different thickness. Because aluminum coating materials were used, the aluminum distribution was considerably observed in coating layer and porous shapes was also observed inside aluminum coating. It is considered that other impurities affect the result because tendency of oxygen was not observed remarkably in porous part. Moreover, Cr was considerably observed which is included in base metal. Therefore, the coating formation is observed by the composition distribution of Cr and Al. In the observation of cross-sections, the laminated microstructure at the coating layer with different thickness was presented. The surface in the case of 90 μm thickness was rough. Overall, similar tendency appeared according to the thickness, but the porosity increased with thickness increasing. In general, the Zn and Al content in the coating layer is known to be approximately 5%-10%. Even though base metal was exposed to seawater environment due to defect like porosity or oxidized substance, base metal could be protected from corrosion. Thermal spray coating brings out sacrificial characteristics if micro-cell forms, because base metal with electrochemically high potential operates as cathode and thermal spray coating layer showing low potential operates as anode. This corrosion protection function is available in the case that thermal spray coating acts as sacrificial anodic. Therefore, the thickness absolutely affects the durability of base metal.

Fig.2 EDX composition distribution of Al coating with different thickness
Fig.3 presents the potential of thermal spray layer in seawater. The potential at early stage shifted to negative direction. For 120 μm thickness coating, the stable potential maintained at about 4 000 s. Thereafter, it shifted slowly to negative direction, and displayed dramatically declination at 57 000 s. Furthermore, for 90 μm thickness coating, it moved to negative direction steadily until 7 000 s and increased to noble potential. The potential of -0.74 V maintained at 20 000 s, and then moved to negative direction continuously until experiment completion. Meanwhile, the potential of 60 μm thickness coating steadily decreased. Overall, the potential presented the higher potential with the increase of coating thickness. It is considered that the noble potential kept up because chloride ion in seawater is hard to penetrate with the thickness increasing, and also the potential with time increasing in thick coating shifted to the negative direction.
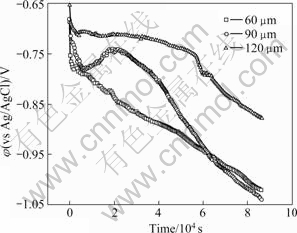
Fig.3 Potential for thermal spray layer with different thickness in seawater
Fig.4 shows the anodic polarization curves of thermal spray coating in seawater. Almost similar trend appeared at around open circuit potential(OCP) and the increase of current density was steadily observed with the increase of potential. The low current density showed at thin coating. In the case of 120 μm thickness coating, small difference was observed from 2.75 V potential. Thereafter, the current density increased with potential increasing constantly. Overall, the characteristics are almost similar, but low current density is observed at thick coatings.

Fig.4 Anodic polarization curves of thermal spray coating in seawater
Fig.5 presented the cathodic polarization curves with different coating thickness in seawater. Like the anodic polarization curves, the similar trend represented at around OCP. The cathodic polarization tendency at all conditions consisted of concentration polarization by dissolved oxygen reduction reaction and activation polarization by hydrogen gas occurrence. Also, in the process of shifting to the negative direction from open circuit potential, the current density increased rapidly in all conditions. Up to approximately -1.25 V, the lowest current density represented at 90 μm thickness coating, but the current density of 120 μm thickness coating represented the lowest value in the below potential. Thereafter, the current density increased with shifting to the negative direction steadily.
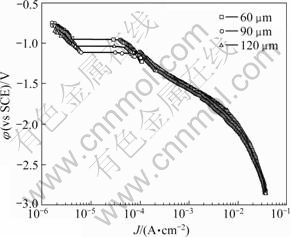
Fig.5 Cathodic polarization curves of thermal spray coating in seawater
Fig.6 displays the time—current density curves of 60 μm thickness coating. At -1 V, in the early stage, the potential moved to the negative direction, then a stable value was observed. The lowest current density displayed. In the potentiostatic experiment performed at 2 V and 4 V, the current density increased rapidly due to the strong active dissolution reaction in anodic polarization curve. In addition, the current density at 2 V presented lower value compared with that at 4 V. This trend is coincided with anodic polarization curve. In the potentiostatic potential experiments at -3 V and -5 V, at -5 V higher value presented. In addition, the current density at 4 V appeared high value compared with that of-5 V, The reason is that activation polarization by dissolution reaction is higher than activation polarization by hydrogen gas occurrence.
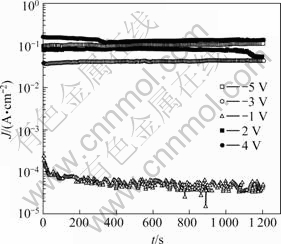
Fig.6 Time—current density curves of 60 μm thickness coating
Fig.7 shows the current density after potentiostatic potential experiment for 1 200 s of thermal spray coating. For all thermal spray coating thickness, the lowest current density displayed at -1 V (around open circuit potential). Also, at -1 V, the highest thickness coating displayed the lowest current density. At 2 V, the coating of 120 μm thickness displayed the highest current density. This is because of the effect of activation polarization by hydrogen gas at cathodic polarization, and by the strong active dissolution reaction at anodic polarization.
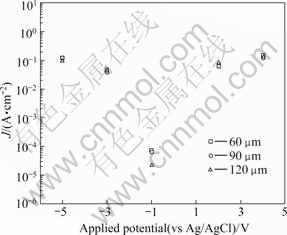
Fig.7 Current density after potentiostatic potential experiment for 1 200 s of thermal spray coating
The photographs of thermal spray coatings after immersion experiment for 1 260 h in seawater are shown in Fig.8. In the whole, the corrosion product in 60 μm thickness coating was observed, and the particle size of corrosion product is the biggest. The corroded area in 90 μm thickness coating was smaller than that of the 60 μm thickness coating, but a big corrosion product was observed in general.
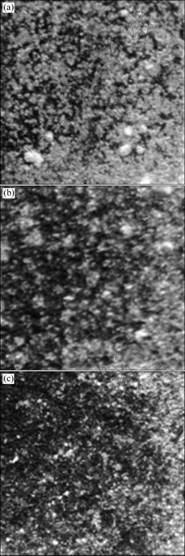
Fig.8 Photographs of thermal spray coating after immersion test for 1 260 h in seawater: (a) 60 μm; (b) 90 μm; (c) 120 μm
However, the traces of dissolution reaction in 120 μm thickness coating appeared, and the corrosion product was observed at restricted area. In conclusion, the corrosion resistance is increased with the coating thickness increasing. There is a result by other researchers that the corrosion product is mainly composed of Al (OH)3[11].
4 Conclusions
If the base metal is exposed to marine environment, the base metal is possible to protect from corrosion due to the sacrificial anode characteristics by forming micro- cell. The base metal operated with the cathode shows electrochemically high potential, and the thermal spray coating layer operated with the anode shows low potential. From the surface and cross-section photographs of thermal spray coating, the large difference difference is not observed at the surface, and in cross- section, porosity was observed due to oxygen or other mixture during the coating processing. From the result of electrochemical experiment, the coating with higher thickness represented good corrosion resistance in seawater.
Reference
[1] KIM S J, WOO Y B, HAN M S, JANG S K, KIM J I. Development of corrosion protection technology by thermal spray coating for STS 304 in sea water [C]//Proceedings of the Korean Marine Engineering Autumn Conference. 2006: 73-74.
[2] KIM S J, WOO Y B, KIM J I. Evaluation of electrochemical characteristics of shaft system materials for small vessel [C]// Proceedings of the Korean Marine Engineering Spring Conference. 2007: 61-62.
[3] JANG S K, KO S C, HAN M S, KIM S J. Characteristics evaluation with coating thickness in Al thermal spray coating for 304 stainless steel [C]//17th World Interfinish Congress and Exposition with 9th ICASE. 2008: 533.
[4] KIM S J, JEONG J Y, KIM J I. Investigation of optimum materials selection in thermal spray coating for the corrosion protection of STS304 [J]. Journal of Ceramic Processing Research, 2007, 8(3): 296-299.
[5] KIM S J, JANG S K, KIM J I. Electrochemical properties and corrosion protection of stainless steel for hot water tank [J]. The Korean Journal of Chemical Engineering, 2004, 21(3): 739-745.
[6] KIM S J, JANG S K, HAN M S. Corrosion protection characteristics in application of various thermal spray coating materials for STS304 [C]//European Materials Research Society, 2007: 117.
[7] KIM S J, LEE S J, WOO Y B, HAN M S, BAIK S Y. Effect of spray distance on corrosion protection characteristics of Al thermal spray coating for stainless steel [C]//Proceedings of the Korean Marine Engineering Autumn Conference. 2008: 217-218.
[8] MCGRANNA R T R, GREVING D J, SHADLEYA J R, RYBICKIA E F, KRUECKEB T L, BODGER B E. The effect of coating residual stress on the fatigue life of thermal spray coated steel and aluminum [J]. Surface and Coatings Technology, 1998, 108/109: 59-64.
[9] TORRES B, CAMPO M, URENA A, RAMS J. Thermal spray coatings of highly reinforced aluminium matrix composites with sol-gel silica coated SiC particles [J]. Surface and Coatings Technology, 2007, 201: 7552-7559.
[10] DESHPANDE S, KULKAMI A, SAMPATH S, HERMAN H. Application of image analysis for characterization of porosity in thermal spray coatings and correlation with small angle neutron scattering [J]. Surface and Coatings Technology, 2004, 187: 6-16.
[11] SUNG J K, LEE S H. Corrosion behavior of thermal spray coating layers in a simulated marine environment [J]. Research Institute of Industrial Science and Technology, 1996, 10(4): 447-462.
Foundation item: Project supported by Honam Sea Grant R&D Program Fund of 2006-2007 Years
Corresponding author: Seok-Ki JANG; Tel: +82-61-2407205; Fax: +82-61-2407201; E-mail: Jangsk@mmu.ac.kr
DOI: 10.1016/S1003-6326(08)60379-9
(Edited by YUAN Sai-qian)