J. Cent. South Univ. (2017) 24: 1013-1018
DOI: 10.1007/s11771-017-3503-z
LiPF6 and lithium difluoro(oxalato)borate/ethylene carbonate+dimethyl carbonate +ethyl(methyl)carbonate electrolyte for LiNi0.5Mn1.5O4 cathode
ZHOU Hong-ming(周宏明)1, 2, GENG Wen-jun(耿文俊)1, LI Jian(李荐)1, 2
1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. Hunan Province Zhengyuan Energy Storage Materials and Devices Research, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Abstract: LiODFB electrolyte’s compatibility with LiNi0.5Mn1.5O4 high-voltage cathode material was studied by cyclic voltammetry, charge-discharge test and AC impedance. The results show that at 25 and 60 °C, the LiODFB-based electrolyte has better electrochemical stability than LiPF6. AC impedance plots show that the LiODFB battery has a lower charge-transfer resistance than LiPF6 battery at 60 °C, which indicates that LiODFB battery has excellent cycling performance at high temperature. At 25 and 60 °C, the LiNi0.5Mn1.5O4/Li half cells with LiODFB or LiPF6 as electrolyte all have simple redox peak, showing that each of them has an excellent reversibility. LiODFB battery has better cycle performance than LiPF6 battery at 25 °C and 60 °C. At 25 °C, their 0.5C initial discharge specific capacities are 126.3 and 131.6 mA·h/g, and their capacity retention ratios of the 100th cycle are 97.1% and 94.7%, respectively. At 60 °C, their 0.5C initial discharge specific capacities are 132.6 and 129.1 mA·h/g, and their capacity retention ratios of the 100th cycle are 94.1% and 81.7%, respectively.
Key words: lithium difluoro(oxalato)borate; LiNi0.5Mn1.5O4; electrochemical performance; compatibility
1 Introduction
Lithium-ion batteries have been widely utilized as a power source for portable electronic devices [1]. Recently, much effort has been made to promote their application in hybrid electric vehicles and dispersed energy storage systems, which demand light weight, small volume, high energy density and highly security [2-4]. As a promising cathode material for lithium ion secondary batteries, LiNi0.5Mn1.5O4 is attracting intensive attention due to its high operation voltage of 4.7 V and its potential to obtain high energy density for next generation batteries [5-7]. However, there is no appropriate electrolyte matched the requirements of high-voltage cathode material. LiPF6 electrolyte will decompose when the working voltage is above 4.5 V, which results in performance degradation of the battery system [8]. Hence, it’s an urgent problem to looking for an electrolyte which has wide electrochemical window.
In 2006, lithium difluoro(oxalato)borate (LiODFB) was first reported by ZHANG [9] as a unique lithium salt for improved electrolyte of lithium-ion battery. Because its chemical structure is similar to LiBOB, a stable solid electrolyte interface (SEI) can be formed on the graphite anode, protecting it from being eroded by solvent, such as propylene carbonate (PC) [10,11]. Furthermore, LiODFB is superior to LiBOB in the properties as below: 1) it is more soluble in linear carbonate solvents that are essential to lower viscosity and provides the probability to optimize the solvents for the higher conductivity over a wide temperature range [12]; 2) it helps to form steady solid electrolyte interface (SEI) with less resistance. Lithium-ion cell using this salt has better power capability and low temperature cyclic performance [13,14]. Besides, LiODFB also has wide electrochemical window, which meets the requirement of the high-voltage cathode material [15]. These unique characteristics make LiODFB as a promising salt for high-power applications.
The previous work about LiODFB was mainly focused on characterization of the reaction on graphite anode, whereas the influences of LiODFB on cathode material, such as LiNi0.5Mn1.5O4, were not reported or elaborated. In this work, we evaluate LiODFB/EC + DMC + EMC electrolyte from the standpoint of the properties that prevent decomposition at high voltage. We also report the significantly improved cycling performance. The results are compared with those of LiPF6.
2 Experimental
2.1 Electrolyte preparation
The electrolytes were prepared by dissolving
1 mol/L LiPF6 or 1 mol/L LiODFB in a blend (1:1:1, w/w/w) of ethylene carbonate(EC), dimethyl carbonate (DMC), and ethyl(methyl) carbonate (EMC). All electrolytes were prepared in an argon-filled glove box (Universal 2440/750, Mikrouna Mech. Tech. Co., Ltd.,Water content: <1×10-6, oxygen content: <1×10-6). Water and free acid contents of the electrolytes were controlled below 20×10-6, which were determined by Mettler Toledo DL32 titrator and Karl-Fisher 798 MPT Titrino, respectively.
2.2 Cell preparation
In order to investigate the electrochemical properties of LiNi0.5Mn1.5O4 cathode in different electrolytes, we assembled the 2032 type coin half-cells in the argon-filled glove box. The working electrode was composed of 80% LiNi0.5Mn1.5O4, 10% carbon black and 10% polyvinylidene fluoride (PVDF). The electrode materials were evenly mixed and then attached on Al foils. The counter and reference electrodes were lithium tablets.
2.3 Cyclic voltammetry and AC impedance measurements
Room temperature (25 °C) and high temperature (60 °C) charge–discharge tests were carried out with the utilization of LiNi0.5Mn1.5O4/Li half-cells. The test cells were cycled using a BTS0105C8 tester between 3.2 V and 5.0 V at 0.5C. A CH instrumental electrochemical workstation (CHI660A) was used to test cyclic voltammetry (CV) and AC impedance for LiNi0.5Mn1.5O4/Li half-cells. CV test was performed with a scan rate of 0.1 mV/s. For the AC impedance, the cells were measured in the frequency range between 20 mHz and 100 kHz with a perturbation amplitude of 5 mV.
2.4 Characterization with scanning electron microscope (SEM)
The cells were dissected after testing at 60 °C, respectively. Their electrodes were taken out. The electrodes were soaked in and rinsed with pure DMC to remove the residual electrolyte. Then, the washed electrodes were dried in a vacuum drier. The electrodes morphologies were observed with SIRION 200 SEM.
3 Results and discussion
3.1 Electrochemical stability window of electrolytes
The electrochemical stability window of the electrolytes at 25 and 60 °C was investigated by the linear sweep voltammetry(LSV) as shown in Figs. 1(a) and (b).
It is evident that the initial decomposition voltages of LiPF6-based electrolyte and LiODFB-based electrolyte at 25 °C were 4.2 V and 5.57 V, respectively (Fig. 1(a)). With further increase of the potential, an obvious peak occurred in each curve. The voltage of the peak of LiDFOB-based electrolyte was 5.87 V, which was higher than that (5.4 V) of LiPF6-based electrolyte. In another word, LiDFOB-based electrolyte had wider electrochemical stability window, which made it more suitable for high voltage battery.
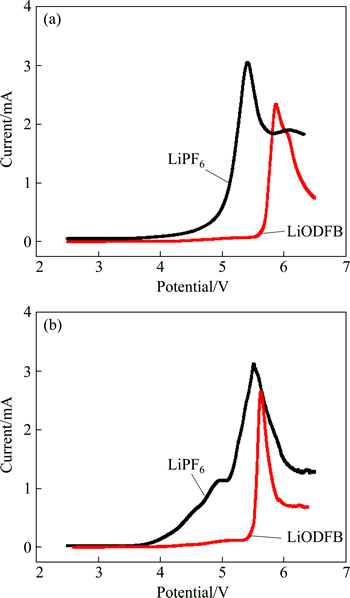
Fig. 1 Linear sweep voltammograms of 1 mol/L LiDFOB (or LiPF6) in 1:1:1 EC/DMC/EMC electrolytes at 25 °C (a) and 60 °C (b) with the scan rate of 1 mV/s (The working and reference electrodes are Pt and lithium, respectively)
At 60 °C, the initial decomposition voltages of LiPF6-based electrolyte and LiODFB-based electrolyte were 3.98 and 4.2 V, respectively. The oxidation current of LiPF6-based electrolyte increased rapidly, which was attributed to the fact that LiPF6 decomposed into HF and LiF when the voltage was around 3.98 V. It was about 5.7 V when the peak appeared which was believed to be solvent oxidization in the LiODFB-based electrolyte. Based on the data above, we can conclude that LiODFB-based electrolyte is more stable at high temperature. Moreover, the decomposition voltages of both kinds of electrolyte decrease with the temperature increasing, which indicates that the stability of electrolytes will get worse when they are working at higher temperature.
3.2 Cyclic voltammetry curves of LiNi0.5Mn1.5O4/Li half-cell
Cyclic voltammetry curves of the LiNi0.5Mn1.5O4/Li half-cell at 25 and 60 °C are plotted in Figs. 2(a) and (b). It can be seen that there is only one peak pair, consisting of one anodic peak and one cathodic peak, which corresponds to the two-phase charge-discharge reaction of the Ni4+/Ni2+ redox couple. At 25 °C, the oxidation and reduction processes of cells using LiODFB as electrolyte salt occurred at 4.45 and 4.97 V, respectively. The corresponding processes of LiPF6-based cell occurred at 4.5 and 4.92 V, respectively. With LiODFB and LiPF6 electrolytes, the voltage separations between the anodic and cathodic peaks were 0.52 and 0.42 V, respectively. At 60 °C, the processes of both kinds of cells were similar to what occurred at 25 °C. The voltage separation can affect the kinetics characteristics at a certain extent. But the difference of the voltage separation between LiODFB-based electrolyte and LiPF6-based electrolyte was small. This indicated that, with LiODFB and LiPF6 electrolytes, LiNi0.5Mn1.5O4/Li half-cells had good reversibility of the reaction. Therefore, both LiDFOB and LiPF6 were friendly to LiNi0.5Mn1.5O4 electrode.
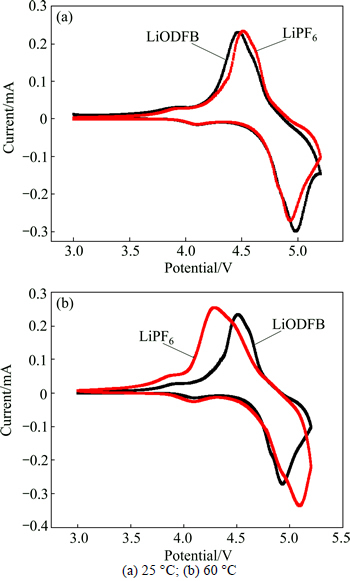
Fig. 2 CV curves of LiNi0.5Mn1.5O4/Li cells at different temperatures:
3.3 Impedance analysis
We attempted to observe AC impedance changes of the cells, and to understand the resistance of the interface film formed on the electrode surface. The cells were measured before and after 100 cycles at 25 and 60 °C, respectively. The impedance spectra of the LiNi0.5Mn1.5O4/Li cells containing the two different electrolytes are shown in Fig. 3.
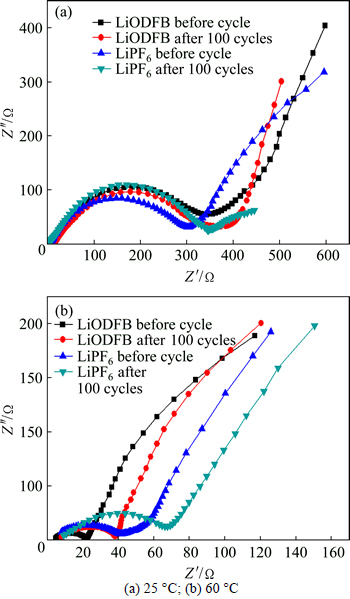
Fig. 3 Electrochemical impedance spectra (EIS) of LiNi0.5Mn1.5O4/Li cell after and before cycle:
All EIS curves consisted of two parts: a semicircle at high and medium frequency, which reflected the charge-transfer reaction resistance Rct, and a sloping line at the low frequency, which gave expression to the diffusion of Li-ion into the active mass. It suggested that the surface film was not formed yet, because of the absence of passive film resistance and its relative capacitance. With the temperature increasing from 25 °C to 60 °C, impedances of all the cells decreased greatly. This was attributed to the increase of conductivity of electrolyte at higher temperature, and the Li-ion moved faster through cathode which made the Li-ion easier to deintercalate.
Figure 3(a) indicates that the electrolyte resistance RL and charge-transfer reaction resistance Rct of LiODFB cell and LiPF6 cell increased respectively after 100 cycles at 25 °C. Figure 3(b) shows that, after 100 cycles, both RL and Rct increased at 60 °C. But due to the instability of LiPF6, the impedance of LiPF6 cell increased obviously. In contrast, because the LiODFB was more stable at 60 °C, the increase of impedance was invisible and much slower. Therefore, though its impedance was a little larger at 25 °C, LiODFB cell had superior cycling performance and small impedance at high temperature.
3.4 Initial charge-discharge performance of LiNi0.5Mn1.5O4/Li half-cell
The initial charging and discharging performance of LiNi0.5Mn1.5O4/Li half-cells in different electrolytes at 25 and 60 °C are compared in Fig. 4. It can be seen that at 25 or 60 °C, LiODFB and LiPF6 cells had much similar voltage-capacity correlations and stable charging and discharging plateau, which meant that LiODFB-based electrolyte had good compatibility with LiNi0.5Mn1.5O4, as well as LiPF6 electrolyte.
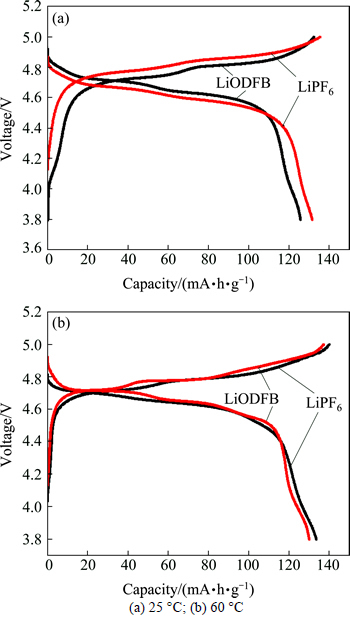
Fig. 4 Initial charge-discharge curves of LiNi0.5Mn1.5O4/Li cells at different temperature:
At 25 °C the initial charge and discharge specific capacities of LiPF6 half-cell were 138.8 and 131.6 mA·h/g, respectively. The corresponding specific capacities of LiODFB half-cell were 134.2 and 126.3 mA·h/g, respectively. The results suggested that there were very similar cycling efficiencies of the first cycle for LiPF6 and LiODFB cells, which were 94.8% and 94.1%, respectively.
At 60 °C the cycling efficiency of LiODFB half-cell was higher than that of LiPF6 half-cell, which indicated that the LiPF6 had the tendency to decompose into HF and LiF. Solid LiF particles may move to the separator with ions and block the micropores to encumber the transport of Li-ion. HF would damage the electrode material, and let the capacity down directly. In comparison, LiDFOB had a little smaller conductivity but much better stability which brought the cell larger capacity. Therefore, we may conclude that at 25 °C both LiDFOB and LiPF6 are suitable for Li-ion cell from the capacity aspect. But LiDFOB half-cell has better performance when the half-cell works at 60 °C.
3.5 Cycling performance of LiNi0.5Mn1.5O4/Li half-cell
The cycle performance of LiNi0.5Mn1.5O4/Li cells with different electrolytes at 25 and 60 °C is shown in Fig. 5. As shown in Fig. 5(a), at 25 °C it can be seen that the LiNi0.5Mn1.5O4/Li cell with LiPF6-based electrolyte initially delivered a discharge specific capacity of 131.6 mA·h/g and retained 120.7 mA·h/g at the 100th cycle. However, when LiODFB was used as the lithium salt in the electrolyte, the LiNi0.5Mn1.5O4/Li cell initially delivered a discharge specific capacity of 126.3 mA·h/g and retained 122.6 mA·h/g at the 100th cycle. At the 100th cycle, the discharge capacity retention for LiODFB was 97.1%, higher than that (91.7%) of LiPF6. Thus, the cycling performance of the cells using LiODFB as lithium salt in electrolyte was better than that of the cells using LiPF6 as lithium salt.
As shown in Fig. 5(b), at 60 °C the LiNi0.5Mn1.5O4/Li cell with LiPF6-based electrolyte initially delivered a discharge specific capacity of 129.8 mA·h/g and retained 106.1 mA·h/g at the 100th cycle. However, when LiODFB was used as the electrolyte, the LiNi0.5Mn1.5O4/Li cell initially delivered a discharge specific capacity of 132.6 mA·h/g and retained 125.6 mA·h/g at the 100th cycle. The discharge capacity retentions were 81.7% and 94.7% for LiPF6 and LiODFB at the 100th cycle, respectively. We can conclude that LiODFB cells had superior cycling performance, especially at elevated temperature, which was attributed to the more effective, compact and stable surface film formed on the LiNi0.5Mn1.5O4 surface. Besides, LiPF6 electrolyte was decomposed at high temperature and voltage easily, which caused the attenuation of capacity for LiPF6 cells. The results suggested that the cell with LiODFB electrolyte was suitable for high temperature and voltage discharge.
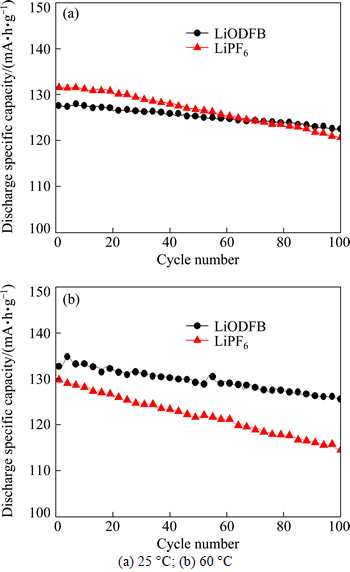
Fig. 5 Cycling performance of LiNi0.5Mn1.5O4/Li batteries at different temperatures:
3.6 Rate performance of LiNi0.5Mn1.5O4/Li half-cell
The cycling performance of LiNi0.5Mn1.5O4/Li half-cells in different electrolyte at different rates are compared in Fig. 6. It can be seen that the specific discharge capacities gradually decreased with increasing the applied current densities for both the half-cells. The specific discharge capacity of half-cell using LiPF6 electrolyte was a little bit higher than that using LiODFB electrolyte at 0.5C, which was due to the higher conductivity of LiPF6. However, at 1C , 2C and 5C the half-cells with LiODFB electrolyte had higher specific discharge capacity, which was due to the more stable SEI film formed on the LiNi0.5Mn1.5O4 surface of LiODFB electrolyte. The results suggested that the half-cell with LiODFB electrolyte was suitable for high-rate discharge.
3.7 SEM analysis
Figure 7 shows the SEM images of the LiNi0.5Mn1.5O4 electrodes taken from the cells with the two electrolytes, which were cycled at 60 °C, respectively. The SEM images of LiNi0.5Mn1.5O4 electrodes cycled at 60 °C with LiPF6-based and LiODFB-based electrolyte are shown in Figs. 7(b) and (c). It can be seen from Fig. 7(c) that after 100 cycles theLiNi0.5Mn1.5O4 particles were corroded and wrapped by some substances. This was attributed to the corrosion by HF (from the decomposition of LiPF6) and the corrosion products. However, the corrosion of LiODFB-based electrolytes on LiNi0.5Mn1.5O4 was slight and the stability was good at 60 °C. Therefore, the images of LiNi0.5Mn1.5O4 particles in LiODFB-based cell after 100 cycles were almost the same as that before cycle. This indicated that LiODFB electrolyte was not able to erode the LiNi0.5Mn1.5O4 electrode.
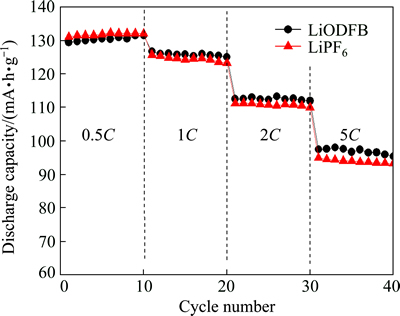
Fig. 6 Cycling performance of LiNi0.5Mn1.5O4/Li batteries at different rates
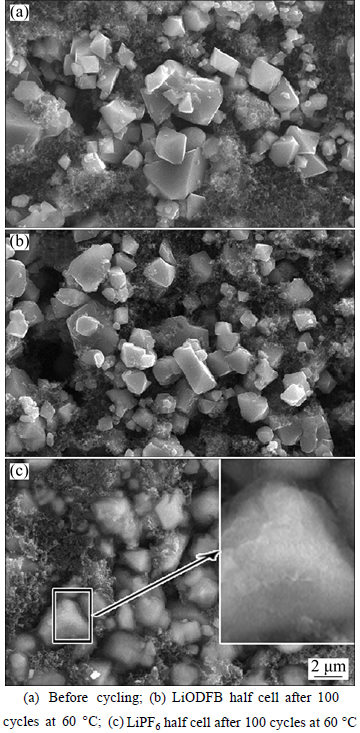
Fig. 7 SEM images of LiNi0.5Mn1.5O4 electrode from LiNi0.5Mn1.5O4/Li cells after and before cycle:
4 Conclusions
1) The LiODFB-based electrolyte had better electrochemical stability than LiPF6-based electrolyte at 25 and 60 °C.
2) At 25 and 60 °C CV and initial cycling curves of LiNi0.5Mn1.5O4/Li half-cell with both LiODFB and LiPF6 electrolytes had single pair of oxidation-reduction peaks and stable charge-discharge plateau. This indicated that both kinds of electrolytes were friendly to LiNi0.5Mn1.5O4 electrode.
3) The increment of impedance of LiODFB cell after 100 cycles at 60 °C was significantly smaller than the increment of LiPF6 cell’s impedance, which showed that the cell with LiODFB electrolyte system was more stable than that with LiPF6 at high temperature.
4) At 25 °C the discharge capacity retentions were 91.7% and 97.1% of LiNi0.5Mn1.5O4/Li half-cell for LiPF6 and LiODFB at the 100th cycle, respectively; at 60 °C the capacity retentions were 81.7% and 94.7% for LiPF6 and LiODFB, respectively. That is to say, the capacity retention would rise by using LiODFB instead of LiPF6.
5) Analyzing the SEM image, we noticed that many of particles of LiNi0.5Mn1.5O4 using LiPF6 as electrolyte salt were corroded after 100 cycles at 60 °C, while using LiPF6 as electrolyte salt the particles of LiNi0.5Mn1.5O4 were almost intact.
References
[1] RITCHIE A, HOWARD W. Recent developments and likely advances in lithium-ion batteries [J]. Journal of Power Sources, 2006, 162(2): 809-812.
[2] MIERLO J V, den BOSSCHE P V, MAGGETTO G. Models of energy sources for EV and HEV: Fuel cells, batteries, ultracapacitors, flywheels and engine-generators [J]. Journal of Power Sources, 2004, 128(1): 76-89.
[3] ARAI J, YAMAKI T, YAMAUCHI S, YUASA T, MAESHIMA T, SAKAI T, KOSEKI M, HORIBA T. Development of a high power lithium secondary battery for hybrid electric vehicles [J]. Journal of Power Sources, 2005, 146(1): 788-792.
[4] BENINATI S, DAMEN L, MASTRAGOSTINO M. Fast sol-gel synthesis of LiFePO4/C for high power lithium-ion batteries for hybrid electric vehicle application [J]. Journal of Power Sources, 2009, 194(2): 1094-1098.
[5] SANTHANAM R, RAMBABU B. Research progress in high voltage spinel LiNi0.5Mn1.5O4 material [J]. Journal of Power Sources, 2010, 195(17): 5442-5451.
[6] KIM M C, KIM S H, ARAVINDAN V, KIM W S, LEE S Y. Ultrathin polyimide coating for a spinel lini0.5mn1.5o4 cathode and its superior lithium storage properties under elevated temperature conditions [J]. Journal of the Electrochemical Society, 2013, 160(8): 1003-1008.
[7] AKLALOUCH M, AMARILLA J M, ROJAS R M, SAADOUNE I, ROJO J M. Chromium doping as a new approach to improve the cycling performance at high temperature of 5 V LiNi0.5Mn1.5O4-based positive electrode [J]. Journal of Power Sources, 2008, 185(1): 501-511.
[8] YANG Xu-lai, WANG Yang, CAO He-kun, XU Xiao-ming. Progress in high-voltage electrolytes for lithium ion batteries [J]. Chinese Journal of Power Sources, 2012, 36(8): 1235-1238. (in Chinese)
[9] ZHANG Sheng-shui. An unique lithium salt for the improved electrolyte of Li-ion battery [J]. Electrochemistry Communications, 2006, 8(9): 1423-1428.
[10] ZHANG S S, XU K, JOW T R. A new approach toward improved low temperature performance of Li-ion battery [J]. Electrochemistry Communications, 2002, 4(11): 928-932.
[11] XU K, ZHANG S S, LEE U, ALLEN J L, JOW T R. LiBOB: Is it an alternative salt for lithium ion chemistry [J]. Journal of Power Sources, 2005, 146(1): 79-85.
[12] FU M H, HUANG K L, LIU S Q, LIU L S, LI Y K. Lithium difluoro (oxalato) borate/ethylene carbonate + propylene carbonate + ethyl(methyl) carbonate electrolyte for LiMn2O4 cathode [J]. Journal of Power Sources, 2010, 195(3): 862-866.
[13] ZHANG Zhi-an, CHEN Xu-jie, LI Fan-qun, LAI Yan-qing, LI Jie, LIU Ping, WANG Xin-yu. LiPF6 and lithium oxalyldifluoroborate blend salts electrolyte for LiFePO4/artificial graphite lithium-ion cells [J]. Journal of Power Sources, 2010, 195(21): 7397-7402.
[14] GAO Hong-quan, ZHANG Zhi-an, LAI Yan-qing, LI Jie, LIU Ye-xiang. Structure characterization and electrochemical properties of new lithium salt LiODFB for electrolyte of lithium ion batteries [J]. Journal of Central South University of Technology, 2008, 15: 830-834.
[15] ZHOU Hong-ming, FANG Zhen-qi, LI Jian. LiPF6 and lithium difluoro(oxalato)borate/ethylene carbonate + dimethyl carbonate + ethyl(methyl)carbonate electrolyte for Li4Ti5O12 anode [J]. Journal of Power Sources,2013, 230: 148-154.
(Edited by DENG Lü-xiang)
Cite this article as: ZHOU Hong-ming, GENG Wen-jun, LI Jian. LiPF6 and lithium difluoro(oxalato)borate/ethylene carbonate+dimethyl carbonate +ethyl(methyl)carbonate electrolyte for LiNi0.5Mn1.5O4 cathode [J]. Journal of Central South University, 2017, 24(5): 1013-1018. DOI: 10.1007/s11771-017-3503-z.
Foundation item: Project (K1403375-11) supported by Science and Technology Planning Project of Changsha, China
Received date: 2015-10-20; Accepted date: 2017-01-11
Corresponding author: LI Jian, Associated Professor, PhD; Tel: +86-731-88877173; E-mail: ziliao2000@126.com