DOI:10.19476/j.ysxb.1004.0609.2017.04.025
双滴共沉淀法合成MgO-LDH的脱氟性能
汪爱河1, 2,周康根1,刘 行1,陈泉洲1,刘 芳1
(1. 中南大学 冶金与环境学院,长沙 410083;
2. 湖南城市学院 市政与测绘工程学院,益阳 413000)
摘 要:采用双滴共沉淀法制备MgO-LDH水滑石去除溶液中氟,并采用静态实验系统地讨论溶液初始pH值、氟初始浓度和吸附时间等因素对MgO-LDH吸附性能影响。结果表明:吸附条件对氟的吸附能力影响较大,适宜的MgO-LDH投加量为10 g/L,溶液初始pH为6.40;随着温度的升高,MgO-LDH的吸附量也随之增加。在较佳的实验条件下,MgO-LDH对氟的最大吸附量为16.60 mg/g。动力学数据分析显示,准二级动力学方程(R2=0.9314~ 0.9907)比准一级动力学方程(R2=0.7941~0.9919)能更好地描述吸附动力学特征。颗粒内扩散方程拟合结果发现,氟在MgO-LDH吸附过程包括表面吸附和颗粒内扩散两个过程。吸附等温数据拟合发现,Langmuir吸附等温式(R2=0.9982~0.9992)比Freundlich吸附等温式(R2=0.6904~0.9453)更好地描述氟在MgO-LDH上的等温吸附行为。
关键词:MgO-LDH;含氟废水;吸附等温线;吸附动力学
文章编号:1004-0609(2017)-04-0869-07 中图分类号:X703.1 文献标志码:A
含氟废水主要来源于半导体行业和冶金工业,排放废水氟浓度从几个到几千mg/L不等,是对水环境安全产生危险因素之一[1]。含氟废水常规处理方法为石灰石和沉淀法,但用该方法出来后的废水氟离子浓度通常难以达到10 mg/L的排放标准,需要进一步的深度处理。吸附法因其操作简单、效率高和成本低等特点而备受环境工作者的青睐[2]。含氟废水常用吸附剂有铝类吸附剂、钙类吸附剂、炭基吸附剂和水滑石。在这些吸附剂,水滑石因其价格低廉、高吸附性和独特结构而被应用到含氟处理[3]。 等[4]以Mg(NO3)2、Al(NO3)3为镁源和铝源,NaOH和Na2CO3为沉淀剂合成LDH,并将其应用到含氟废水处理,取得较好的吸附效果。CAI等[5]采用共沉淀法合成Mg-Al-CO3型LDH对含磷和氟废水进行处理,结果显示,合成的LDH对磷和氟的最大吸附量分别为0.63 mmol/g和1.42 mmol/g。WU等[6]合成Fe-Al-Ce型LDH处理含氟废水,结果显示含氟废水的去除主要采用的是和金属离子形成金属氟化物的形式。文献[7-10]中报道的合成LDH方法均采用硝酸盐类作为镁源和铝源,NaOH和Na2CO3为沉淀剂,LDH合成成本高,制约LDH吸附法的实际应用。本文作者采用双滴共沉淀法,以廉价的氯化铝作为铝源,氧化镁为镁源,NaOH和Na2CO3为沉淀剂合成LDH,并将其应用到含氟废水的处理中,探讨MgO-LDH对氟离子吸附动力学和等温吸附特征。
等[4]以Mg(NO3)2、Al(NO3)3为镁源和铝源,NaOH和Na2CO3为沉淀剂合成LDH,并将其应用到含氟废水处理,取得较好的吸附效果。CAI等[5]采用共沉淀法合成Mg-Al-CO3型LDH对含磷和氟废水进行处理,结果显示,合成的LDH对磷和氟的最大吸附量分别为0.63 mmol/g和1.42 mmol/g。WU等[6]合成Fe-Al-Ce型LDH处理含氟废水,结果显示含氟废水的去除主要采用的是和金属离子形成金属氟化物的形式。文献[7-10]中报道的合成LDH方法均采用硝酸盐类作为镁源和铝源,NaOH和Na2CO3为沉淀剂,LDH合成成本高,制约LDH吸附法的实际应用。本文作者采用双滴共沉淀法,以廉价的氯化铝作为铝源,氧化镁为镁源,NaOH和Na2CO3为沉淀剂合成LDH,并将其应用到含氟废水的处理中,探讨MgO-LDH对氟离子吸附动力学和等温吸附特征。
1 实验
1.1 主要试剂
盐酸、氢氧化钠、氧化镁、氯化铝、氟化钠、氯化钠、碳酸钠、冰乙酸和二水柠檬酸钠均为分析纯。
1.2 MgO-LDH的合成
按照镁铝摩尔比4:1分别称取氧化镁40 g、氯化铝60.375 g;将氯化铝溶解在300 mL蒸馏水中配置成盐溶液;氧化镁直接加入三口烧瓶,并加入400 mL水溶解;称取氢氧化钠80 g、碳酸钠13.25 g溶解在300 mL蒸馏水配置成碱溶液,用蠕动泵将盐溶液和碱溶液加入到三口烧瓶中,控制三口烧瓶的pH值为9~10;在60 ℃晶化12 h,过滤,在80 ℃烘烤24 h,得到产品研磨粒径小于74 μm后制成吸附剂,记为MgO-LDH。合成实验装置图如图1所示。

图1 MgO-LDH合成实验装置示意图
Fig. 1 Schematic diagram of experimental apparatus for preparing MgO-LDH
1.3 吸附剂投加量对吸附的影响
取6个100 mL带盖塑料瓶,加入pH≈6的含氟50 mg/L模拟废水100 mL,分别加入0.2、0.4、0.6、0.8、1.0、2.0 g吸附剂,放置到水浴振荡器中,并设置水浴振荡器温度为303 K、振荡速度400 r/min;振荡24 h,取上清液经0.22 μm过滤,用氟离子选择电极测溶液氟离子浓度。
1.4 pH和吸附时间对脱氟的影响
将500 mL、50 mg/L的含氟模拟废水加入1 L烧杯中,分别设置初始pH为5、6、7、9、11,加入5 g吸附剂;设置温度为303 K并在磁力搅拌器搅拌,每隔一定时间(10、30、50、110、170、290、720、1440 min)取样经0.22 μm过滤,测定氟离子浓度。
1.5 氟离子初始浓度和反应温度对脱氟的影响
将100 mL、pH≈6含氟模拟废水加入100 mL塑料瓶中,分别设置初始氟浓度为20、40、60、120、240、360 mg/L,加入1 g吸附剂;设置温度为303 K,并在水浴振荡器振荡24 h取样经0.22 μm过滤测定氟离子浓度。
1.6 分析方法
用X射线衍射仪(D/max2550VB+,日本理学株氏会社)对样品结构进行表征,Cu靶,Kα辐射源,λ=0.15406 nm,管电压36 kV,管电流30 mA,扫描速率8 (°)/min,10°~80°扫描;样品形貌特征由场发射扫描电子显微镜(Nano SEM 230,FEI公司)拍摄观察,工作电压5 kV,溶液中氟离子采用氟离子选择电极进行分析。
2 结果与讨论
2.1 MgO-LDH性能表征
图2所示为MgO-LDH的XRD谱。从图2可看出,MgO-LDH具有非常尖锐的特征性衍射峰,谱图基线平稳、衍射峰窄且强度极高,说明合成的MgO-LDH结晶度高、规整性好。合成MgO-LDH出现类水滑石特征峰[11-12]:(003)峰(0.754 nm)、(006)峰(0.377 nm)、(012)峰(0.255 nm)、(110)峰(0.151 nm)。
图3所示为MgO-LDH的SEM像和EDX谱。由图3可以看出,合成的MgO-LDH表现为结晶形态良好,但颗粒有些团聚。EDX分析表明,MgO-LDH表面由Mg、Al、C、O、Cl元素组成,镁铝摩尔比为2.26:1,比制备中比例略低。

图2 MgO-LDH的XRD谱
Fig. 2 XRD pattern of MgO-LDH

图3 吸附剂MgO-LDH的SEM像和EDX谱
Fig. 3 SEM image(a) and EDX spectrum(b) of MgO-LDH adsorbent
2.2 投加量对脱氟的影响
向100 mL的含氟模拟废水中投加一定量的MgO-LDH,在水浴振荡器上振荡24 h后测定溶液中氟含量,MgO-LDH投加量与除氟效果关系如图4所示。
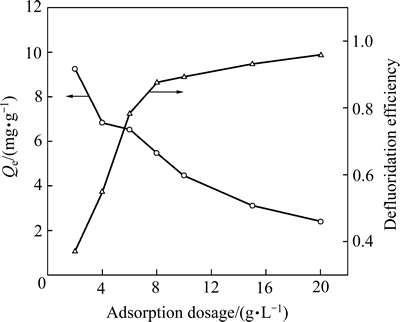
图4 吸附剂投加量对脱氟的影响
Fig. 4 Effect of adsorption dosage on defluoridation efficiency
从图4可以看出,脱氟率受投加量的影响较大;当投加量从2 g/L增加到10 g/L,溶液脱氟率也在增加,投加量为10 g/L,吸附达到最佳,为85.06%;当投加量超过10 g/L,脱氟率变化不大。出现这种现象主要原因:1) 吸附剂量的增加使单位吸附剂上的吸附位增多;2) 投入过多的吸附剂会相互聚集,致使表面吸附位降低,氟单位吸附量反而下降。兼顾考虑脱氟率与单位质量吸附剂的吸附量,MgO-LDH投加量以10 g/L为宜。
2.3 pH和吸附时间对脱氟的影响
溶液pH主要影响分子离解成离子或络合状态的程度,同时也影响吸附剂表面形态及电荷特性。为研究pH对MgO-LDH脱氟影响,实验设置不同溶液初始pH,结果如图5所示。
由图5可知,随着溶液初始pH增加,溶液中氟去除率也在减小,当溶液pH≤6.40,MgO-LDH对氟吸附24 h后,脱氟率达到0.75以上;而当溶液pH>6.40时,吸附24 h后,脱氟率不到50%。这主要原因[13]:1) 在酸性条件下,质子化作用使得MgO-LDH表面带正电荷,更容易吸附带负电的氟离子;2) 在碱性条件下,溶液中OH-浓度增大,与氟离子产生竞争,使MgO-LDH对氟离子的脱氟率减少。

图5 初始pH对脱氟的影响
Fig. 5 Effect of initial pH on defluoridation efficiency
2.4 温度和氟初始浓度对脱氟的影响
氟初始质量浓度和温度对MgO-LDH吸附的影响如图6所示。由图6可以看出,氟初始浓度从20 mg/L增加到360 mg/L时,MgO-LDH对氟的吸附量在不断增大。这是由于氟初始浓度的增加,加大氟与MgO-LDH吸附点位结合的概率。所以,随着初始浓度的增加,吸附容量逐渐增大。另随温度升高,吸附量也都增大,表明MgO-LDH对氟的吸附是吸热反应,升温有利于反应进行。

图6 不同温度下氟初始浓度对脱氟的影响
Fig. 6 Effect of initial fluoride concentration on defluoridation at different temperatures
2.5 吸附等温线拟合
MgO-LDH对氟的吸附过程可以通过吸附等温线来描述,吸附平衡后吸附量和吸附平衡浓度的关系如图7所示,采用Langmuir等温吸附方程式[14](1)和Freundlich等温吸附方程式[15](2)对吸附数据进行拟合,拟合参数见表1。
Langmuir等温吸附方程:
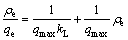 (1)
(1)
Freundlich等温吸附方程:
 (2)
(2)
式中: 为吸附平衡后溶液中的氟质量浓度,mg/L;qe为吸附平衡后单位质量吸附剂对氟的吸附量,mg/g;kL为Langmuir等温吸附方程常数,L/mg;kF为Freundlich等温吸附方程常数,mg/g。
为吸附平衡后溶液中的氟质量浓度,mg/L;qe为吸附平衡后单位质量吸附剂对氟的吸附量,mg/g;kL为Langmuir等温吸附方程常数,L/mg;kF为Freundlich等温吸附方程常数,mg/g。
从表1可以看出,Langmuir吸附等温方程能够较好地描述MgO-LDH对氟的吸附过程。这表明MgO-LDH表面吸附电位均匀分布,且其对氟离子为单层吸附。使用Langmuir吸附等温方程对氟离子的等温吸附进行拟合(R2>0.99),在氟离子初始质量浓度为20~360 mg/L、吸附温度为303~333 K的条件下,其最大饱和吸附量为15.06~17.06 mg/g。且在303、313、323和333 K下,Langmuir吸附等温方程kL为正值,且随着温度的上升,kL增大。这表明随温度上升,MgO-LDH对氟的吸附能力增强,且该吸附反应是自发进行的。Freundlich吸附等温方程常数kF和n分别代表吸附能力和吸附量随浓度增加的强度,可表示吸附反应进行的难易程度。一般认为,n<0.5表示吸附反应难以进行,n为2~10表示吸附反应容易进行,且n越大,表示吸附反应越容易进行,吸附效果越好。在不同温度下,MgO-LDH对F-吸附的Freundlich方程常数n均大于2,表明以氧化镁为镁源合成LDH具有较好地吸附性能。从表2可以看出,合成的MgO-LDH吸附材料较大多数材料具有较高的氟吸附性能。

图7 MgO-LDH脱氟等温吸附曲线
Fig. 7 Defluoridation isotherms adsorption curves of MgO-LDH
表1 MgO-LDH吸附等温线方程的参数及相关系数
Table 1 Adsorption isotherms equation parameters and coefficients of MgO-LDH

表2 不同吸附剂对氟吸附能力的比较
Table 2 Comparison of adsorption capacities of different adsorbents for fluoride

2.6 吸附动力学拟合
常用准一级动力学方程、准二级动力学方程和颗粒内扩散模型来描述吸附剂对吸附质的吸附动力学特征,其线性方程如表3所示。MgO-LDH对氟的吸附动力学拟合结果如图8和表4所示。
表3 MgO-LDH吸附动力学方程
Table 3 Adsorption kinetic equations of MgO-LDH

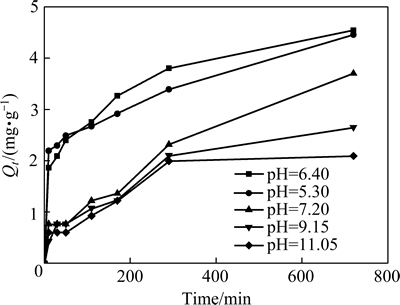
图8 初始pH和吸附时间对吸附量的影响
Fig. 8 Effect of initial pH and adsorption time on adsorption amount by MgO-LDH
从图8和表4可以看出,准一级动力方程和准二级动力方程对MgO-LDH的吸附数据均能较好地拟合,但准一级动力学方程对MgO-LDH最大饱和吸附量的预测误差要较准二级动力学方程大,说明MgO-LDH的吸附动力学特征应采用准二级动力学方程描述更合适些。这主要是准一级动力学方程的缺陷,在进行数据拟合时需要知道平衡吸附量,而达到平衡吸附需要很长时间,故拟合度较低。另外,颗粒内扩散模型数据计算表明(R2=0.8802~0.9890),该吸附反应也受到颗粒内扩散速率的限制,但其边界常数C不为0,因此,颗粒内扩散速率不是MgO-LDH对氟吸附反应唯一限制因素,还受到液膜扩散、表面吸附等影响。
3 结论
1) 以氯化铝为铝源、氧化镁为镁源,利用双滴共沉淀法合成MgO-LDH吸附材料对氟有较好吸附性能;在pH为6.40,投加量为10 g/L,温度333 K条件下对氟的吸附量可达16.60 mg/g。
2) MgO-LDH对氟吸附过程符合Langmuir吸附等温式,其拟合相关系数均大于0.99,最大理论吸附量为17.06 mg/g。
3) 随着吸附时间的增加,MgO-LDH对氟吸附量也随之增加,在吸附300 min后基本达到平衡,且吸附动力学特征可用可以用准二级动力学模型描述,其相关系数为0.9530~0.9907。颗粒内扩散速率也是其吸附反应限制因素,但不是唯一限制因素。
表4 不同初始pH时MgO-LDH吸附动力学参数
Table 4 Kinetic model parameters for adsorption of fluoride onto MgO-LDH at different initial pH

REFERENCES
[1] 蒋云福, 仁付莉, 唐 源, 周婉莹, 马 薇, 廖 洋, 赵仕林.改性腐殖酸去除水中氟化物性能研究[J]. 安全与环境学报. 2014, 14(6): 190-197.
JIANG Yun-fu, REN Fu-li, TANG Yuan, ZHOU Wan-ying, MA Wei, LIAO Yang, ZHAO Shi-lin. On the increased efficiency of fluorine removal from water with the modified humic acid[J]. Journal of Safety and Environment, 2014, 14(6): 190-197.
[2] RAJKUMAR S, MURUGESH S, SIVASANKAR V, DARCHEN A, MSAGATI T A M, CHAABANE T. Low-cost fluoride adsorbents prepared from a renewable biowaste syntheses, characterization and modeling studies[J]. Arabian Journal of Chemistry, 2015, 126(3): 1250-1399.
[3] KAMEDA T, OBA J, YOSHIOKA T. Kinetics and equilibrium studies on Mg-Al oxide for removal of fluoride in aqueous solution and its use in recycling[J]. Journal of Environmental Management, 2015, 156: 252-256.
[4]  Liang, HE Jing, WEI Min, EVANS D G, DUAN Xue. Factors influencing the removal of fluoride from aqueous solution by calcined Mg-Al-CO3 layered double hydroxides[J]. Journal of Hazardous Materials, 2006, 133(1/3): 119-128.
Liang, HE Jing, WEI Min, EVANS D G, DUAN Xue. Factors influencing the removal of fluoride from aqueous solution by calcined Mg-Al-CO3 layered double hydroxides[J]. Journal of Hazardous Materials, 2006, 133(1/3): 119-128.
[5] CAI Peng, ZHENG Hong, WANG Chong, MA Hong-wen, HU Jian-chao, PU Yu-bing, LIANG Peng. Competitive adsorption characteristics of fluoride and phosphate on calcined Mg-Al-CO3 layered double hydroxides[J]. Journal of Hazardous Materials, 2012, 213/214(2): 100-108.
[6] WU Xiao-mei, ZHANG Yu, DOU Xiao-min, ZHAO Bei, YANG Min. Fluoride adsorption on an Fe-Al-Ce trimetal hydrous oxide: Characterization of adsorption sites and adsorbed fluorine complex species[J]. Chemical Engineering Journal, 2013, 223: 364-370.
[7] 程 翔, 黄新瑞, 王兴祖, 孙德智. ZnAlLa类水滑石对污泥脱水液中磷酸根的吸附[J]. 化工学报, 2010, 61(4): 955-962.
CHENG Xiang, HUANG Xin-rui, WANG Xing-zu. Phosphate adsorption by ZnAlLa layered double hydroxides from excess sludge filtrate[J]. Ciesc Journal, 2010, 64(4): 955-963.
[8] 倪哲明, 王巧巧, 姚 萍, 刘晓明, 李 远. Mg/Al水滑石的焙烧产物吸附酸性红88的动力学和热力学机理研究[J]. 化学学报, 2011, 69(5): 529-535.
NI Zhe-ming, WANG Qiao-qiao, YAO Ping, LIU Xiao-ming, LI Yuan. Kinetics and thermodynamics for acid red 88 adsorption on calcined Mg/Al layered double hydroxides[J]. Acta Chimica Sinica, 2011, 69(5): 529-535.
[9] 于 洋, 岳秀萍, 刘吉明, 曹 岳. Mg/Al/Fe型类水滑石焙烧产物吸附去除水中硫酸根离子[J]. 环境工程学报, 2013(8): 3079-3084.
YU Yang, YUE Xiu-ping, LIU Ji-ming, CAO Yue. Removal of sulfate ions from aqueous solution via adsorption on calcined Mg/Al/Fe hydrotalcite-like compounds[J]. Chinese Journal of Environmental Engineering, 2013, 7(8): 3079-3084.
[10] 王红宇, 刘 艳. 类水滑石Mg/Zn/Al焙烧产物对高氯酸盐的吸附[J]. 环境科学, 2014, 35(7): 2585-2589.
WANG Hong-yu, LIU Yan. Adsorption of perchlorate by calcined Mg/Zn/Al layered double hydroxides[J]. Environmental Science, 2014, 35(7): 2585-2589.
[11] JIAO F, SHUAI L, YU J, JIANG X, CHEN X, DU S. Adsorption of glutamic acid from aqueous solution with calcined layered double Mg-Fe-CO3 hydroxide[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(12): 3971-3978.
[12] ZHANG Fen, ZHANG Chang-lei, SONG Liang, ZENG Rong-chang, LIU Zhen-guo, CUI Hong-zhi. Corrosion of in-situ grown MgAl-LDH coating on aluminum alloy[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(10): 3498-3504.
[13] DHILLON A, NAIR M, BHARGAVA S K, KUMAR D. Excellent fluoride decontamination and antibacterial efficiency of Fe-Ca-Zr hybrid metal oxide nanomaterial[J]. Journal of Colloid and Interface Science, 2015, 457: 289-297.
[14] WANG Yun-yan, YAO Wen-bin, WANG Qing-wei, YANG Zhi-hui, LIANG Li-fen, CHAI Li-yuan. Synthesis of phosphate-embedded calcium alginate beads for Pb(Ⅱ) and Cd(Ⅱ) sorption and immobilization in aqueous solutions[J]. Transactions of Nonferrous Metals Society of China, 2016, 26(8): 2230-2237.
[15] ABDELWAHAB N A, AL-ASHKAR E A, EL-GHAFFAR M A A. Preparation and characterization of eco-friendly poly(p-phenylenediamine) and its composite with chitosan for removal of copper ions from aqueous solutions[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(11): 3808-3819.
[16] ASGARI G, ROSHANI B, GHANIZADEH G. The investigation of kinetic and isotherm of fluoride adsorption onto functionalize pumice stone[J]. Journal of Hazardous Materials, 2012, 217/218: 123-132.
[17] MUTHU PRABHU S, MEENAKSHI S. Synthesis of surface coated hydroxyapatite powders for fluoride removal from aqueous solution[J]. Powder Technology, 2014, 268: 306-315.
[18] LIANG Peng, ZHANG Yi, WANG Dong-feng, XU Ying, LUO Lan. Preparation of mixed rare earths modified chitosan for fluoride adsorption[J]. Journal of Rare Earths, 2013, 31(8): 817-822.
[19] SWAIN S K, PATNAIK T, SINGH V K, JHA U, PATEL R K, DEY R K. Kinetics, equilibrium and thermodynamic aspects of removal of fluoride from drinking water using meso-structured zirconium phosphate[J]. Chemical Engineering Journal, 2011, 171(3): 1218-1226.
[20] LEYVA-RAMOS R, RIVERA-UTRILLA J, MEDELLIN- CASTILLO N A, SANCHEZ-POLO M. Kinetic modeling of fluoride adsorption from aqueous solution onto bone char[J]. Chemical Engineering Journal, 2010, 158(3): 458-467.
[21] MAITI A, BASU J K, DE S. Chemical treated laterite as promising fluoride adsorbent for aqueous system and kinetic modeling[J]. Desalination, 2011, 265(1/3): 28-36.
[22] XU Xiao-tian, LI Qin, CUI Hao, PANG Jian-feng, SUN Li, AN Hao, ZHAI Jian-ping. Adsorption of fluoride from aqueous solution on magnesia-loaded fly ash cenospheres[J]. Desalination, 2011, 272(1/3): 233-239.
[23] BABAEIVELNI K, KHODADOUST A P. Adsorption of fluoride onto crystalline titanium dioxide: Effect of pH, ionic strength, and co-existing ions[J]. Journal of Colloid and Interface Science, 2013, 394: 419-427.
[24] 喻 清, 丁德馨, 李登科, 余园平, 罗 艺, 王启方, 胡 南. 固定化黑曲霉活性炭吸附铀的机理[J]. 中国有色金属学报, 2016, 26(4): 936-945.
Yu Qing, Ding De-xin, Li Deng-ke, Yu Yuan-ping, Luo Yi, Wang Qi-fang, Hu Nan. Adsorption mechanism of uranium of immobilizing Aspergillus niger activated carbon[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(4): 936-945.
[25] 杨明珠, 李耀威, 王 刚, 刘 健. 氨基硫脲改性硅胶对Pd2+的动态吸附性能[J]. 中国有色金属学报, 2014, 24(7): 1927-1932.
Yang Ming-zhu, Li Yao-wei, Wang Gang, Liu Jian. Dynamic adsorption behavior of Pd2+ on silica gel functionalized with thiosemicarbazide[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(7): 1927-1932.
[26] 方 帅, 刘志强, 陈 瑜, 霍明昕, 边德军, 杨 霞, 耿 直, 朱遂一. 铁泥溶剂热法制备磁性材料及其在水溶液中对亚甲基蓝的吸附性[J]. 中国有色金属学报, 2015, 25(4): 1109-1115.
Fang Shuai, Liu Zhi-qiang, Chen Yu, Huo Ming-xin, Bian De-jun, Yang Xia, Geng Zhi, Zhu Sui-yi. Synthesis of magnetic materials by solvothermal method with iron mud and adsorption of methylene blue in aqueous solution[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(4): 1109-1115.
Deflouridation efficiency of MgO-LDH prepared by double drop co-precipitation
Wang Ai-he1, 2, Zhou Kang-gen1, Liu Xing1, Chen Quan-zhou1, Liu Fang1
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. School of Municipal and Mapping Engineering, Hunan City University, Yiyang 413000, China)
Abstract: The magnesium oxide layer double hydrotalcite (MgO-LDH) was prepared by double drop co-precipitation using magnesium oxide as magnesium source. And it was applied for the treatment of wastewater containing fluoride. The influences of initial fluoride concentration, pH and adsorption time on fluoride adsorption using MgO-LDH were investigated by the batch experiments. The experimental results show that the adsorption capacity for fluoride is affected by the adsorption conditions. The suitable adsorbent dosage is 10 g/L, the suitable pH of MgO-LDH fluorine adsorption is 6.40. The amount of adsorption increase with increasing the temperature. The adsorption capacity of the adsorbent for Fluoride is 16.60 mg/g at optimal conditions. The kinetic data show that the pseudo-second-order model (R2=0.9314-0.9907) can better describe the characteristic of the adsorption kinetic than the pseudo-first-order model (R2=0.7941-0.9919). The results from the Intra-particle model also show that there exist two separate stages in sorption process, which are external diffusion and the diffusion of inter-particle. Langmuir and Freundlich isotherms were used to fit the adsorption equilibrium data, and it is found that the adsorption process follows preferably the Langmuir isotherm adsorption model, which indicates that the adsorption mainly occurs in active region on the surface of MgO-LDH and belong to the monolayer adsorption.
Key words: MgO-LDH; wastewater containing fluoride; adsorption isotherm; adsorption kinetic
Foundation item: Project(2010ZX07212-008) supported by the Key Project of Science and Technology of National Water Pollution Contral and Management, China
Received date: 2016-03-18; Accepted date: 2016-10-20
Corresponding author: Zhou Kang-gen; Tel: +86-13873189654; E-mail: zhoukg63@163.com
(编辑 李艳红)
基金项目:水体污染控制与治理科技重大专项(2010ZX07212-008)
收稿日期:2016-03-18;修订日期:2016-10-20
通信作者:周康根,教授,博士;电话:13873189654;E-mail: zhoukg63@163.com