
J. Cent. South Univ. (2019) 26: 3457-3469
DOI: https://doi.org/10.1007/s11771-019-4266-5
Zircon U-Pb-Hf isotopes and mineral chemistry of Early Cretaceous granodiorite in the Lunggar iron deposit in central Lhasa, Tibet Y, China
ZHANG Yun-hui(张云辉)1, 2, 3, WANG Yang-shuang(王杨双)3, WANG Wen-shu(王文澍)1,LIU Jie(刘洁)1, YUAN Ling-ling(袁玲玲)1
1. Key Laboratory of Metallogenic Prediction of Nonferrous Metals and Geological Environment Monitoring, Ministry of Education, School of Geoscience and Info-physics, Central South University,Changsha 410083, China;
2. Faculty of Geosciences and Environmental Engineering, Southwest Jiaotong University,Chengdu 611756, China;
3. State Key Laboratory of Geohazard Prevention and Geoenvironment Protection, College of Environment and Civil Engineering, Chengdu University of Technology, Chengdu 610059, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: The Lunggar iron deposit belongs to the Bangong-Nujiang metallogenic belt and is located in central Lhasa on the Tibetan Plateau. In the Lunggar deposit, iron mineralization formed in the skarnization contact zone between the Early Cretaceous granodiorite and the late Permian Xiala Formation limestone. In this study, we achieved detailed zircon U-Pb-Hf isotopes and mineral chemistry for the Early Cretaceous granodiorite. Zircon U-Pb dating results indicate that the Early Cretaceous granodiorite emplaced at ca. 119 Ma. Based on the trace elements in zircons and the mineral chemical composition of amphibole and biotite, the Early Cretaceous granodiorite was believed to form under condition of high temperature (>700 °C), low pressure (100-400 MPa), and relatively high oxygen fugacity (lgfO2)(-13.6 to -13.9) and H2O content (4%-8%). Zircon trace elements, Hf isotope and biotite chemistry collectively reveal that significant juvenile mantle-derived magmas contributed to the source of the granodiorite. The relatively high logfO2 and shallow magma chamber are beneficial for skarn iron mineralization, implying remarkable potential for further prospecting in the Lunggar iron deposit.
Key words: zircon U-Pb-Hf isotope; mineral chemistry; crystallization condition; Lunggar iron deposit; central Lhasa
Cite this article as: ZHANG Yun-hui, WANG Yang-shuang, WANG Wen-shu, LIU Jie, YUAN Ling-ling. Zircon U-Pb-Hf isotopes and mineral chemistry of Early Cretaceous granodiorite in the Lunggar iron deposit in central Lhasa, Tibet Y, China [J]. Journal of Central South University, 2019, 26(12): 3457-3469. DOI: https://doi.org/10.1007/s11771- 019-4266-5.
1 Introduction
Due to the multiple subduction of the Tethyan Oceans, extensive magmatism and related mineralization were developed in the Tibetan Plateau [1-3], generating abundant Cu-Mo-Fe-Pb-Zn-Au polymetallic resources [4-6]. To date, a number of Mesozoic iron deposits have been reported, including the Caima, Fuye and Lunggar deposits of the Bangong-Nujiang metallogenic belt, and the Nixiong deposit of the Gangdese metallogenic belt (Figure 1) [7-9].
As a newly-discovered skarn iron deposit, the Lunggar deposit has average ore grade of 59.36%-63.46%, and is the prospecting target area in the Bangong-Nujiang metallogenic belt [9]. FEI et al [9] preliminarily investigated the characteristics of the iron deposit and considered the Early Cretaceous granodiorite an ore-related pluton. CAO et al [8] further addressed the genesis of the multi-stage magmatisms developed in the Lunggar iron deposit and attributed the iron mineralization to the break-off of the Meso-Tehyan Bangong-Nujiang Ocean plate. However, previous studies largely concentrated on the petrogenesis of the granitoids. Sparse knowledge has been achieved to discuss the crystallization conditions of magmas (e.g., temperature, pressure and oxygen fugacity), which are the key factors controlling the enrichment of metal sulfides. Meanwhile, the relationships between the ore-related granodiorite and iron mineralization and the prospecting potential have yet to be investigated.

Figure 1 Tectonic map of China, showing location of Tibet [7] (Map No. GS20161595) (a), tectonic subdivision of Tibet Plateau, showing major sutures and location of Mesozoic iron deposits [8] (b) and geological map of Lunggar iron deposit [9] (c) (JSSZ-Jinshajiang Suture Zone; LSSZ-Longmu Tso-Shuanghu Suture Zone; BNSZ-Bangong-Nujiang Suture Zone; SNMZ-Shiquan River-Nam Tso Mélange Zone; LMF-Luobadui-Milashan Fault LMF-Indus-Yarlung Zangbo Suture Zone; NQ-Northern Qiangtang; SQ-Southern Qiangtang; NL-Northern Lhasa; CL-Central Lhasa; SL-Southern Lhasa)
In this work, we present results from zircon U-Pb-Hf isotopes, in an attempt to clarify the formation age and origin of the granodiorite and the iron mineralization. Moreover, the physico- chemical conditions (oxygen fugacity, pressure and temperature) of the mineralization system are reconstructed, based on a systematic study of zircon trace elements and the chemistry of minerals from the ore-related granodiorite. Combined with the regional magmatic and metallogenic events, the analytical results in this study would be helpful in evaluating the prospecting potential for Lunggar skarn iron deposits.
2 Geological setting and petrography
The Tibetan Plateau consists of the Songpan-Ganzi flysch complex, Qiangtang terrane, Lhasa terrane and Himalaya terrane (Figure 1(b)). These terranes are separated by the Jinshajiang Suture Zone (JSSZ), Bangong-Nujiang Suture Zone (BSZ) and Indus-Yarlung Zangbo Suture Zone (IYZSZ) from north to south, respectively [10]. Furthermore, the Qiangtang terrane can be subdivided into North Qiangtang and South Qiangtang by Longmu Tso-Shuanghu Suture Zone, while the Lhasa terrane can be subdivided into northern Lhasa, central Lhasa and southern Lhasa by the Shiquan River-Nam Tso Mélange Zone (SNMZ) and Luobadui-Milashan Fault (LMF) [11].
Northern Lhasa and Southern Lhasa are composed of juvenile basement, whereas central Lhasa features ancient basement. The basement of northern Lhasa and central Lhasa are covered by Middle Triassic to Cretaceous sedimentary rocks and by Carboniferous-Permian and Upper Jurassic-Lower Cretaceous sedimentary rocks, respectively. Extensive Cretaceous-Palaeogene Gangdese batholiths and Palaeogene Linzizong volcanic rocks with scarce sedimentary strata are distributed in southern Lhasa.
3 Deposit geology
The Lunggar iron deposit is located in western central Lhasa (Figure 1(b)). In the deposit, the sedimentary strata consist of the late Permian Xiala Formation limestone and Quaternary alluvial sediments (Figure 1(c)). Several periods of magmatism are evident in the deposit, including Early Cretaceous granodiorite in the south and Late Cretaceous diorite in the west with scattered Early Cretaceous granite porphyry and early Palaeogene mafic dykes (Figure 1(c)). Alterations, such as skarnization, carbonatization, chloritization and weak sericitization are developed in the country rock. The contact zone between the late Permian Xiala Formation limestone and Early Cretaceous granodiorite is the main host for the metalliferous skarn, forming two primary ore bodies in the west. Ore minerals contain magnetite (>80%) and minor limonite and hematite, while gangue minerals include calcite, diopside, chlorite and epidote.
The Early Cretaceous granodiorite is grey to white and presents the texture of medium to coarse grain with massive structure. It is comprised by plagioclase (40%-46% in volume), quartz (18%-23%), alkali feldspar (15%-19%), amphibole (10%-14%), biotite (5%-8%) and accessory minerals (e.g., titanite, apatite, zircon, and Fe-Ti oxides). The chemical compositions of plagioclase, amphibole, and biotite analysed from the Lunggar granodiorite are provided in Table 1. Biotites consist of 35.00%-36.65% SiO2 and 9.50%-9.99% MgO, and are characterized as ferro-biotite (Figure 3(a)). Plagioclases are prismatic and have Or contents of 22%-34%, belonging to the anorthoclase classification (Figure 3(b)). Amphiboles with subhedral to euhedral crystals present high CaB values of 1.78-1.85 (>1.5), high (Na+K)A values of 0.59-0.70 (>0.5), and low Ti values of 0.13-0.14 (<0.5), and are edenite (Figure 3(c)).
4 Sampling and methods
Fifteen samples were acquired from different fresh outcrops of the Lunggar granodiorite. Samples LGR-1, larger than 5 kg, was used for U-Pb zircon dating and Hf isotope analysis. Fourteen samples were chosen for mineral chemical analysis of plagioclase, biotite and amphibole.
Zircons for U-Pb dating and Hf isotope analyses were separated from crushed whole-rock samples by following standard density and magnetic separation techniques and then purified by handpicking under a binocular microscope. Prior to analysis, all zircons were documented with transmitted and reflected light micrographs as well as cathodoluminescence (CL) images to identify their internal complexities. Zircon U-Pb analysis was done using the laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) at the State Key Laboratory of Geological Processes and Mineral Resources (SKLGPMR), China University of Geosciences, Beijing, China. Zircon 91500 was used as an external standard for calibration [14]. Data reduction and concordia diagram creation were performed using the ICPMSDataCal software [15] and Isoplot 3.7 software [16]. See Ref. [1] for more details.

Figure 2 Field photos (a, b) and photomicrographs (c, d) from granodiorite in Lunggar deposit:(crossed-polarized light) (Amp-amphibole, Bt-biotite, Pl-plagioclase, Qtz-quartz)
Table 1 Electron microprobe analyses of selected minerals from Lunggar granodiorite (Unit: wt%; “-”=below detection)
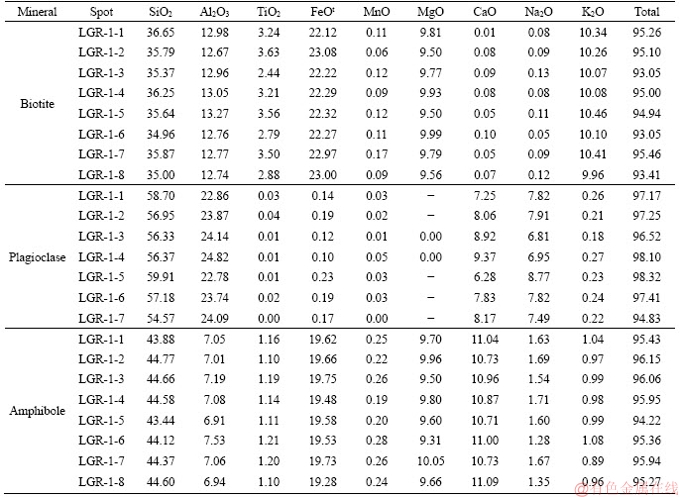
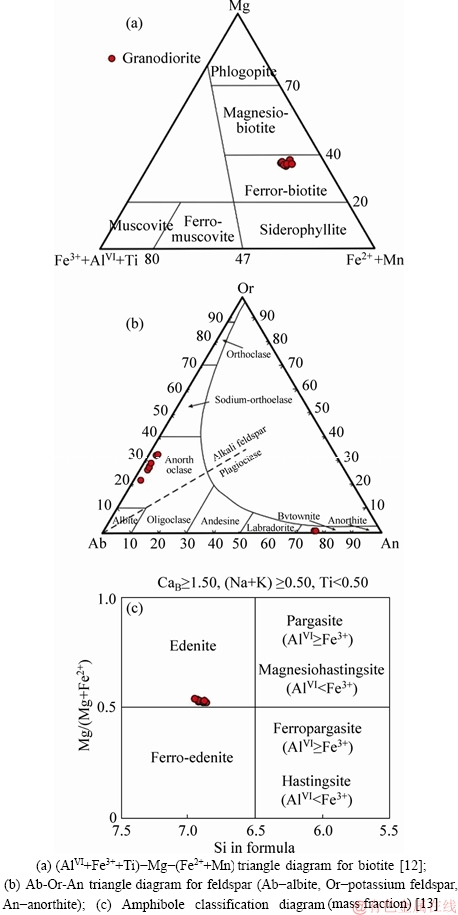
Figure 3 Geochemical characteristics of minerals from Lunggar granodiorite:
Zircon Hf isotope analysis was carried out using Neptune Plus MC-ICPMS at the SKLGPMR, China University of Geosciences, Wuhan, China. The GJ-1 zircon was chosen as the standard for error correction. More detailed processes follow those documented by HOU et al [17]. The 176Hf/177Hf ratios for the GJ-1 standard was 0.282015±8 (2σ, n=10), consistent with the recommended values from ELHLOU et al [18]. See Ref. [1] for more details.
Mineral analysis was carried out using a JEOL JXA-8100 electron microprobe at Chang’an University, China. The operating conditions included a 15 kV accelerating voltage, 10 nA beam current and 1 μm spot diameter. Data reduction followed the ZAF correction procedure. The detection limits of the measured oxides were less than 0.01%. The errors were lower than 5%.
5 Zircon U-Pb-Hf isotopes
LA-ICP-MS U-Pb zircon dating and Hf isotope results are listed in Tables 2-4 and presented in Figure 4. Zircon grains primarily exhibit similar morphological characteristics. Zircon grains are typically euhedral and have prismatic structures, showing crystal lengths from 120 to 260 μm with length-to-width ratios from 2:1 to 3:1 (Figure 4(a)). The zircon grains display colourless and transparent characters with oscillatory zoning in CL images. The U and Th contents of the zircon spots vary from 210×10-6 to 775×10-6 and 138×10-6 to 516×10-6, respectively. Zircons are characterized by relatively high with Th/U ratios (0.6-1.2), positive Ce anomalies (Ce/Ce*=3.13-246.26) and negative Eu anomalies (Eu/Eu*=0.03-0.42) (Figures 5(a) and (b)). All the features above consistently suggest a magmatic origin [19]. Based on the ratios between (Nb/Pb)N and Eu/Eu*, the zircon grains mainly plot the area between the I- and S-type granites (Figure 5(c)). The Nb/Yb and U/Yb ratios of zircon grains are indicative of continental arc magmatism (Figure 5(d)).
Table 2 LA-ICP-MS zircon U-Pb isotopic analyses for zircon grains from Lunggar granodiorite

Table 3 Trace elements (10-6), crystallization temperatures (°C) and magma oxygen fugacity of zircons from Lunggar granodiorite
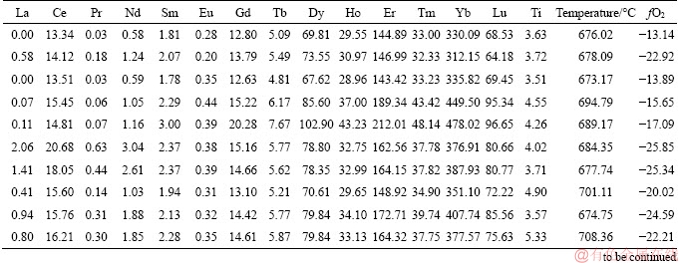
Continued

Table 4 Analytical results of zircon Hf isotopes for Lunggar granodiorite
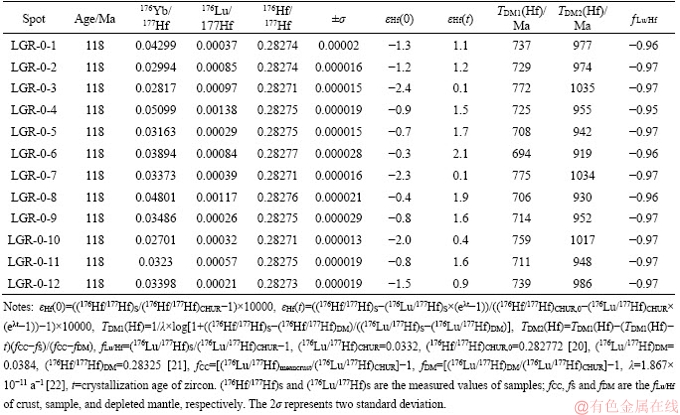
Twenty-five plots concentrated on those zircon grains from sample LGR-1. Twenty-two analyses with concordant ages yield a 206Pb/238U weighted mean age of (119±2) Ma (mean standard weighted deviation, MSWD=2.3). The remaining three grains give discordant ages. Twelve zircons from the granodiorite (calculated at ca.119 Ma) yield slightly positive initial εHf(t) values of +0.1 to +2.1, corresponding to juvenile Hf crustal model ages (TDM2) of 919-1035 Ma.
6 Discussion
6.1 Physico-chemical crystallization conditions
6.1.1 Geothermobarometry for temperature and pressure
The temperature and pressure of crystallization can clarify the formation process of igneous rocks, while the depth of the source can be calculated by establishing the formation temperature and pressure of the magma [27-30]. Currently, experimental petrology information has proven to be efficient for estimating the original temperature and pressure of granitic magma. For instance, the mineral chemical composition of amphibole, biotite and plagioclase are used as robust geobarometers to investigate the temperature and pressure conditions.

Figure 4 Cathodoluminescence (CL) images of representative zircon grains (a), and concordia diagrams of granodiorite (b, c)

Figure 5 Chondrite-normalized REE patterns (a), Ce/Ce* versus (Sm/La)N (N denotes normalization to chondrite values, [13]) (b) (Nb/Pb)N versus Eu/Eu* [23] (c) and lg(Nb/Yb) versus lg(U/Yb) diagrams (d) [24] for the Lunggar granodiorite (Eu/Eu*=2*w(Eu)N/[w(Sm)N+w(Gd)N], Ce/Ce*=2*w(Ce)N/[w(La)N+w(Pr)N]; N denotes the normalized to chondritic values of [25])
Due to the close correlation between the total Al content of hornblende and pressure, various geobarometers have been employed to calculate the pressure of crystallization. However, many previous geobarometers have been shown to be inconsistent compared to experimental results. Therefore, RIDOLFI et al [30] constructed new thermobarometers based on a summary of available experimental data. Herein, according to the new thermobarometric formulation, the crystallization temperatures of the granodiorite range from 787 to 794 °C.
The amphibole geobarometers were applied to estimating the pressure and corresponding depth for the magmatic rocks. The new thermo-barometric equation proposed by RIDOLFI et al [27, 28] was first carried out on volcanic and experimental amphiboles, and then extended to plutonic amphiboles recently. Assuming an average density of 2700 kg/m3 for the upper crust, the crystallization pressure and corresponding depth were calculated. The calculated results suggest a crystallization pressure of 110-120 MPa and corresponding depths of 4.4 to 4.7 km.
The Ti-in-biotite thermometer was also used to estimate the crystallization temperatures of biotite [31]. The calculated temperatures for the Lunggar granodiorite range from 736 to 779 °C. In addition, some classical diagrams have been plotted to approximately estimate the condition of crystallization including temperature and pressure. The biotite Ti versus Mg/(Mg+Fe) diagram and biotite-amphibole P-T diagram indicate that the granodiorite formed under condition of 690-750 °C and 100-400 MPa, consistent with the calculated results from the amphibole geobarometers (Figure 6)[31, 32]. Meanwhile, crystallization temperatures of the granitoids can be achieved using the Ti content in zircon. As such, the results calculated by Ti-in-zircon thermometry imply that the Lunggar granodiorite was crystallized at 673-769 °C.
6.1.2 Oxygen fugacity and H2O content
The chemical compositions of mafic silicate minerals are significantly affected by oxygen fugacity (fO2) at a given fH2O and total pressure. For instance, significant negative correlations exist between the Fe/(Fe+Mg) ratios of these silicates and fO2 values [33]. In this study, the composition of amphibole and biotite from granodiorite can be used to assess the redox conditions of Early Cretaceous magmatism in the Lunggar deposit.

Figure 6 Ti versus Mg/(Mg+Fe) for biotite [31] (a), amphibole Ti/(Mg+Fe+Ti+Mn) vs biotite Ti/(Mg+Fe+ Ti+Mn) diagram (b) and amphibole Al/(Al+Mg+Fe+Ti+ Mn+Si) vs biotiteAl/(Al+Mg+Fe+Ti+Mn+Si) [32] diagrams (c)
The Fe/(Fe+Mg) ratios of amphiboles from the granodiorite are 0.52-0.54. Based on amphibole Fe/(Fe+Mg) vs AlIV diagrams, it can be interpreted that amphiboles in the granodiorite crystallized under transitional conditions between medium- and high-fO2 [33] (Figure 7(a)). The Mg content of amphibole is acceptable to calculate the fO2 according to the methods from RIDOLFI et al [34]. The results show that logfO2 ranges from NNO +0.1 to NNO +0.4. The calculated logfO2 values range from -13.6 to -13.9, indicating relatively high oxygen fugacity. All the samples plot in the field between the biotite Fe2SiO4-Fe3O4 and Ni-NiO (NNO) buffers (Figure 7(b)) [35]. Therefore, the Lunggar granodiorite was crystallized under the medium- to high-fO2 conditions.

Figure 7 Amphibole Fe/(Fe+Mg) versus AlIV diagram [34], showing possible oxygen fugacity conditions during crystallization (a) and biotite Fe3+-Fe2+-Mg triangle diagram [35] (b)
The incorporated Ce4+, and thus the Ce anomalies in zircon grains can be used to evaluate magma oxidation states. A calibration raised by TRAIL et al [36] was used to estimate oxygen fugacity of magmatic melt, shown as below:
ln(Ce/Ce*)=(0.1156±0.0050)×ln(fO2)+(13860±708)/T-6.125±0.484
where fO2 is oxygen fugacity, T is absolute temperature (calculated by Ti-in-zircon thermometry), and Ce/Ce* is the Ce anomaly. In this study, twenty-one analytical results yield oxygen fugacity values ranging from -10.10 to -16.89 with an average of -14.20.
Due to the close relationship between the AlVI content of amphiboles and water concentrations, the AlVI contents of amphibole are available to estimate the stability field of amphibole crystallization [34]. Herein, the water contents estimated for the amphiboles from the granodiorite have a range of 4.3 wt%-4.8 wt%.
6.2 Implications for tectonic and mineralization processes
The magmatism and mineralization in the Lunggar deposit occurred at approximately ca.119 Ma, close to the flare-up of magmatism and related metallogeny in central Lhasa [3]. Previous studies proposed that Early Cretaceous magmatic rocks were products from the subduction of the Banong-Nujiang Ocean [10]. In this study, zircon trace elements indicate that the Lunggar granodiorite was typical of I-type granite emplaced in a continental arc (Figures 5(c) and (d)). It is generally accepted that the mineral chemistry of ferromagnesian minerals (e.g., biotite) is valid for decoding the petrogenesis and tectonic setting of granitic rocks [26]. The chemical composition of biotite is reliable to constrain the sources of magmas. In the biotite FeOt/(FeOt+MgO) versus MgO diagram, the plots of granodiorite are distributed in the area of crustal source(Figure 8(a)) [37]. Major compositions (i.e., FeO, MgO and Al2O3) of biotite in igneous rocks can crystallize from different magmas generated in different tectonic settings. The granodiorite shows calc- alkaline I-type orogenic characteristic in the biotite MgO-FeOt-Al2O3 triangle diagram (Figure 8(b)). The crystallization temperature (>700 °C) and pressure (100-400 MPa) achieved from this study suggest the granodiorite of ca.119 Ma formed under relatively high temperature and shallow depth conditions. In addition, the Hf isotope analyses for the granodiorite yield εHf(t) values of +0.1 to +2.1 and TDM2 ages of 919 to 1037 Ma, indicating significant contribution from juvenile magmas.

Figure 8 Biotite FeOt/(FeOt+MgO) versus MgO [37] (a) and MgO-FeOt-Al2O3 triangle (b) [38] diagrams (FeOt indicates total iron as FeO)
Considering the Bangong-Nujiang Oceanic slab break-off at about 115 Ma, large volumes of hot mantle-derived magmas with abundant metal elements would arise through the “slab window”, providing beneficial conditions for the enrichment of metal elements (e.g., Cu, Fe, Au). The oxygen fugacity of the magma plays a fundamentally important role in the formation of porphyry- and skarn-type metal-sulfide deposits [37]. Under the condition of relatively high oxygen fugacity, the sulfur in the melt remains as S6+ (SO42-) rather than S2- and therefore prevents the generation of sulfide melt or sulfide minerals at depth, producing remarkable potential for the metals to be carried up to shallow crystallization sites to form economic ore bodies. In addition, the destabilization of mantle sulfides would benefit from high fO2 in shallow chambers. Therefore, metal elements (e.g., Cu, Fe and Au) hosted in sulfides would be released and then enriched as ore deposits. In this study, the fO2 is relatively high, benefiting enrichment in Fe. Moreover, PIRAJNO [39] also noted that skarn-type mineralization is more favourable at shallow site (30-300 MPa), consistent with that of the Lunggar granodiorite (100-400 MPa). Combined with the mineralogical characteristics, the magmatism and mineralization are suggested to have developed in a shallow site by the upwelling of hot mantle-derived magmas during oceanic slab break-off, representing relatively great potential for future prospecting.
6 Conclusions
This study presents in detail the zircon U-Pb-Hf isotopes and mineral (biotite, amphibole and plagioclase) chemistry of the Early Cretaceous ore-related granodiorite in the Lunggar iron deposit of central Lhasa. The following conclusions can be drawn:
1) In the Lunggar deposit, the iron mineralization occurred in the skarn zone between the Early Cretaceous granodiorite and late Permian Xiala Formation limestone. Zircon U-Pb dating results reveal that the Early Cretaceous ore-related granodiorite developed at ca. 119 Ma, while iron mineralization occurred slightly later than ca.119 Ma.
2) During the Bangong-Nujiang Oceanic slab break-off, the granodiorite involving significant juvenile mantle-derived magmas formed under conditions of high temperature and shallow depth.
3) High temperature and fO2 and shallow depth can surely permit us to conclude that the Lunggar iron deposit shows relatively high potential for prospecting, as well as a beneficial metallogenic setting in central Lhasa.
References
[1] ZHANG Y H , CAO H W , HOLLIS S P, TANG L, XU M, JIANG J S, GAO S B, WANG Y S. Geochronology, geochemistry and Sr-Nd-Pb-Hf isotopes of the Early Paleogene gabbro and granite from Central Lhasa, southern Tibet: Petrogenesis and tectonic implications [J]. International Geology Review, 2019, 61(7): 868-894.
[2] ZHENG Y C, FU Q, HOU Z Q, YANG Z S, HUANG K X, WU C D, SUN Q Z. Metallogeny of the northeastern Gangdese Pb–Zn–Ag–Fe–Mo–W polymetallic belt in the Lhasa terrane, southern Tibet [J]. Ore Geology Reviews, 2015, 70: 510-532.
[3] HOU Z Q, DUAN L F, LU Y J, ZHENG Y C, ZHU D C, YANG Z M, YANG Z S, WANG B D, PEI Y R, ZHAO Z D, MCCUAIG T C. Lithospheric Architecture of the Lhasa Terrane and its control on ore deposits in the Himalayan-Tibetan Orogen [J]. Economic Geology, 2015, 110(6): 1541-1575.
[4] WANG R, WEINBERG R F, COLLINS W J, RICHARDS J P, ZHU D C. Origin of postcollisional magmas and formation of porphyry Cu deposits in southern Tibet [J]. Earth-Science Reviews, 2018, 181: 122-143.
[5] SUN X, ZHENG Y Y, XU J, HUANG L H, GUO F, GAO S B. Metallogenesis and ore controls of Cenozoic porphyry Mo deposits in the gangdese belt of Southern Tibet [J]. Ore Geology Reviews, 2017, 81: 996-1014.
[6] DUAN J J, TANG J X, LIN B. Zinc and lead isotope signatures of the Zhaxikang PbZn deposit, South Tibet: Implications for the source of the ore-forming metals [J]. Ore Geology Reviews, 2016, 78: 58-68.
[7] CAO H W, HUANG Y, LI G M, ZHANG L K, WU J Y, DONG L, DAI Z W, LU L. Late Triassic sedimentary records in the northern Tethyan Himalaya: Tectonic link with Greater India [J]. Geoscience Frontiers, 2018, 9(1): 273-291.
[8] CAO H W, ZHANG Y H, SANSTOSH M, LI G M, HOLLIS S P, ZHANG L K, PEI Q M, TANG L, DUAN Z M. Petrogenesis and metallogenic implications of Cretaceous magmatism in Central Lhasa, Tibetan Plateau: A case study from the Lunggar Fe skarn deposit and perspective review [J]. Geological Journal, 2019, 54(4): 2323-2346.
[9] FEI F, YANG Z S, LIU Y C, ZHAO X Y, YU Y S. Petrogenetic epoch of the rock mass in the Lunggar iron deposit of Coqen County, Tibet [J]. Acta Petrologica et Mineralogica, 2015, 34: 568-580. (in Chinese)
[10] PAN G T, WANG L Q, Li R S, YUAN S H, JI W H, YIN F G, ZHANG W P, WANG B D. Tectonic evolution of the Qinghai-Tibet Plateau [J]. Journal of Asian Earth Sciences, 2012, 53: 3-14.
[11] ZHU D C, ZHAO Z D, Niu Y L, MO X X, CHUNG S L, HOU Z Q, WANG L Q, WU F Y. The Lhasa Terrane: Record of a microcontinent and its histories of drift and growth [J]. Earth and Planetary Science Letters, 2011, 301(1, 2): 241-255.
[12] FOSTER M D. Interpretation of the composition of trioctahedral micas [J]. Geological Survey Professional Paper, 1960, 354: 11-49.
[13] LEAKE B E, WOOLLEY A R, BIRCH W D, ERNST A J, FERRARIS G, GRICE J D, HAWTHORNE F C, KISCH H J, KRIVOVICHEV V G, SCUMACHER J C, STEPHENSON N C N, WHITTAKER E J W. Nomenclature of amphiboles: Additions and revisions to the International Mineralogical Association’s 1997 recommendations [J]. The Canadian Mineralogist, 2003, 41(6): 1355-1362.
[14] WIEDENBECK M, HANCHAR J M, PECK W H. Further characterisation of the 91500 zircon crystal [J]. Geostandards and Geoanalytical Research, 2004, 1: 9-39.
[15] LIU Y S, HU Z C, ZONG K Q, GAO C G, GAO S, XU J, CHEN H H. Reappraisement and refinement of zircon U-Pb isotope and trace element analyses by LA-ICP-MS [J]. Chinese Science Bulletin, 2010, 55(15): 1535-1546.
[16] GAO Y, WEI R H, MA P X, HOU Z Q, YANG Z S. Post-collisional ultrapotassic volcanism in the Tangra Yumco-Xuruco graben, south Tibet: Constraints from geochemistry and Sr–Nd–Pb isotope [J]. Lithos, 2009, 110(1-4): 129-139.
[17] HOU K J, LI Y H, ZOU T R, QU X M, SHI Y R, XIE G Q. Laser ablation-MC-ICP-MS technique for Hf isotope microanalysis of zircon and its geological applications: Acta Petrologica Sinica, 2007, 23: 2595-2604. (in Chinese)
[18] ELHLOU S, BELOUSOVA E, GRIFFIN W L, PEARSON W L, O’REiLLY S Y. Trace element and isotopic composition of GJ-red zircon standard by laser ablation [J]. Geochimica et Cosmochimica Acta, 2006, 70(18, Supplement): A158.
[19] CORFU F, HANCHAR J M, HOSKIN P W, KINNY P. Atlas of zircon textures [J]. Reviews in Mineralogy and Geochemistry, 2003, 53: 469-500.
[20] BLICHERT-TOFT J, ALBAREDE F. The Lu-Hf isotope geochemistry of chondrites and the evolution of the mantle- crust system [J]. Earth and Planetary Science Letters, 1997, 148(1): 243-258.
[21] GRIFFIN W L, PEARSON N J, BELOUSOVA E, JACKSON S E, van ACHTERBERGH E, O'REILLY S Y, SHEE S R. The Hf isotope composition of cratonic mantle: LAM-MC-ICPMS analysis of zircon megacrysts in kimberlites-Kimberlites and related rocks [J]. Geochimica et Cosmochimica Acta, 2000, 64(1): 133-147.
[22] SODERLUND U, JONATHAN P P, VERVOORT J D, ISACHSEN C E. The 176Lu decay constant determined by Lu-Hf and U-Pb isotope systematics of Precambrian mafic intrusions [J]. Earth and Planetary Science Letters, 2004, 219(3, 4): 311-324.
[23] HOSKIN P W. Trace-element composition of hydrothermal zircon and the alteration of Hadean zircon from the Jack Hills, Australia [J]. Geochimica Et Cosmochimica Acta, 2005, 69: 637-648.
[24] WANG Q, ZHU D C, ZHAO Z D, GUAN Q, ZHANG X Q, SUI Q L, MO X X. Magmatic zircons from I-, S- and A-type granitoids in Tibet: Trace element characteristics and their application to detrital zircon provenance study [J]. Journal of Asian Earth Sciences, 2012, 53: 59-66.
[25] GRIMES C B, WOODEN J L, CHEADLE M J, JOHN B E. “Fingerprinting” tectono-magmatic provenance using trace elements in igneous zircon [J]. Contributions to Mineralogy and Petrology, 2015, 170: 1-26.
[26] ESFAHANI M M, KHALILI M, BAKHSHI M. Petrogenesis of Soheyle-Pakuh and Golshekanan granitoid based on mineral chemistry of ferromagnesian minerals (north of Nain), Iran [J]. Journal of African Earth Sciences, 2017, 129: 973-986.
[27] LI X W, MO X X, SCHELTENS M, GUAN Q. Mineral chemistry and crystallization conditions of the Late Cretaceous Mamba pluton from the eastern Gangdese, Southern Tibetan Plateau [J]. Journal of Earth Science, 2016, 27(4): 545-570.
[28] ZHANG J Q, LI S R, SANTOSH M, WANG J Z, LI Q. Mineral chemistry of high-Mg diorites and skarn in the Han-Xing Iron deposits of South Taihang Mountains, China: Constraints on mineralization process [J]. Ore Geology Reviews, 2015, 64: 200-214.
[29] CAO H W, ZHANG Y H, SANTOSH M, ZHANG S T, TANG L, PEI Q M. Mineralogy, zircon U-Pb-Hf isotopes, and whole-rock geochemistry of Late Cretaceous-Eocene granites from the Tengchong terrane, western Yunnan, China: Record of the closure of the Neo-Tethyan Ocean [J]. Geological Journal, 2018, 53(4): 1423-1441.
[30] RIDOLFI F, RENZULLI A, PUERINI M. Stability and chemical equilibrium of amphibole in calc-alkaline magmas: an overview, new thermobarometric formulations and application to subduction-related volcanoes [J]. Contributions to Mineralogy and Petrology, 2010, 1: 45-66.
[31] HENRY D J, GUIDOTTI C V, THOMSON J A. The Ti-saturation surface for low-to-medium pressure metapelitic biotites: Implications for geothermometry and Ti-substitution mechanisms [J]. American Mineralogist, 2005, 2: 316-328.
[32] CHEN G Y, SUN D S, ZHOU X R, SHAO W, GONG R T, SHAO Y. Genetic mineralogy and gold mineralization of Guojialing granodiorite in Jiaodong region [M]. China University of Geosciences Press, 1993: 107-140. (in Chinese)
[33] ANDERSON J L, SMITH D R. The effects of temperature and fO2 on the Al-in-hornblende barometer [J]. American Mineralogist, 1995, 5-6: 549.
[34] TRAIL D, WATSON E B, TAILBY N D. The oxidation state of Hadean magmas and implications for early Earth’s atmosphere [J]. Nature, 2011, 480: 79-82.
[35] WONES D, EUGSTER H. Stability of biotite-Experiment theory and application [J]. American mineralogist, 1965, 9: 1228.
[36] RICHARDS J P. The oxidation state, and sulfur and Cu contents of arc magmas: Implications for metallogeny [J]. Lithos, 2015, 233: 27-45.
[37] ZHOU Z X. The origin of instrusive mass in Fengshadong, Hubei Province [J]. Acta Petrologica Sinica, 1986, 2: 59-70. (in Chinese)
[38] ABDELRAHMAN A. Nature of biotites from alkaline, calc-alkaline, and peraluminous magmas [J]. Journal of Petrology, 1994, 35(2): 1025-1029.
[39] PIRAJNO F. Hydrothermal Processes and Mineral Systems [M]. Berlin: Springer. 2009.
(Edited by FANG Jing-hua)
中文导读
西藏中拉萨地块隆格尔铁矿床含矿花岗闪长岩锆石U-Pb-Hf同位素和矿物化学组分研究
摘要:隆格尔铁矿床隶属班公湖-怒江成矿带,位于青藏高原的中拉萨地块内。在隆格尔矿床中,铁矿化发育于早白垩系花岗闪长岩和晚二叠系下拉组灰岩的矽卡岩化接触带上。本文报道了地早白垩系花岗闪长岩(119 Ma)的锆石U-Pb-Hf同位素和矿物化学组分特征。基于锆石微量元素和角闪石、黑云母的化学组分,计算了岩体形成时的压力、温度、氧逸度和水成分。结果显示,早白垩系花岗闪长岩形成于高温(>700 °C)、低压(100-100 MPa)、高氧逸度(-13.6~-13.9)的环境中。锆石Hf同位素和黑云母化学组分显示,花岗闪长岩中有明显年轻幔源成分混入。较高的氧逸度和浅的形成环境有利于铁矿化的形成,说明隆格尔铁矿床具有较好的找矿潜力。
关键词:锆石U-Pb-Hf同位素;矿物化学组分;结晶环境;隆格尔铁矿床;中拉萨
Foundation item: Project(2018YSJS14) supported by the Open Research Fund Program of Key Laboratory of Metallogenic Prediction of Nonferrous Metals and Geological Environment Monitoring (Central South University), Ministry of Education, China
Received date: 2018-12-27; Accepted date: 2019-05-08
Corresponding author: YUAN Ling-ling, PhD, Associated Professor; Tel: +86-15874195130; E-mail: yuanll@csu.edu.cn; ORCID: 0000-0002-5907-857X