医用钛合金Ti-3Zr-2Sn-3Mo-25Nb表面自组装抗凝血复合涂层的构建及其血液相容性
来源期刊:中国有色金属学报(英文版)2012年第12期
论文作者:余 森 于振涛 韩建业 WANG Gui 牛金龙 Matthew S. Dargusch
文章页码:3046 - 3052
关键词:Ti-3Zr-2Sn-3Mo-25Nb钛合金;血液相容性;肝素化;表面改性; 静电自组装
Key words:Ti-3Zr-2Sn-3Mo-25Nb alloy; haemocompatibility; heparinization; surface modification; electrostatic self assembly
摘 要:为了提高新型近β钛合金Ti-3Zr-2Sn-3Mo-25Nb(TLM)的抗凝血性能,先通过优化溶胶-凝胶技术在TLM合金试样表面制备出一层致密、均匀的TiO2薄膜,再结合表面官能化处理和静电层自组装技术,在TiO2薄膜表面构建一个多层肝素功能涂层。通过XRD分析改性处理后TLM合金表面涂层的相组成,借助 SEM、AFM研究功能涂层的表面形貌和微观特征,通过动态凝血时间、溶血率、血小板粘附实验对TLM合金表面改性前后的抗凝血性能和血液相容性进行评估。结果表明,经表面肝素化修饰处理后,TLM合金试样表面光滑、平整,同时材料表面的动态凝血时间延长,溶血率明显降低,试样表面粘附的血小板很少且没有被明显激活,TLM合金的抗凝血性能和血液相容性均得到了明显的改善。
Abstract: The haemocompatibility of Ti-3Zr-2Sn-3Mo-25Nb biomedical alloy was studied after surface heparinization. A layer of sol-gel TiO2 films was applied on the alloy samples followed by active treatment in the bio-functionalized solution for introducing the OH- and groups, and then the heparin was immobilized on the active TiO2 films through the electrostatic self assembly technology. It is shown that the heparinized films are mainly composed of anatase and rutile with smooth and dense surface. In vitro blood compatibility was evaluated by haemolysis test, clotting time and platelet adhesion behavior tests. The results show that the haemocompatibility of the alloy could be significantly improved by surface heparinization.
Trans. Nonferrous Met. Soc. China 22(2012) 3046-3052
YU Sen1, YU Zhen-tao1, HAN Jian-ye1, WANG Gui 2, NIU Jin-long1, Matthew S. Dargusch2
1. Northwest Institute for Nonferrous Metal Research, Xi’an 710016, China;
2. CAST Cooperative Research Centre, School of Mechanical and Mining Engineering, The University of Queensland, Brisbane QLD 4072, Australia
Received 26 September 2011; accepted 9 November 2012
Abstract: The haemocompatibility of Ti-3Zr-2Sn-3Mo-25Nb biomedical alloy was studied after surface heparinization. A layer of sol-gel TiO2 films was applied on the alloy samples followed by active treatment in the bio-functionalized solution for introducing the OH- and 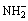 groups, and then the heparin was immobilized on the active TiO2 films through the electrostatic self assembly technology. It is shown that the heparinized films are mainly composed of anatase and rutile with smooth and dense surface. In vitro blood compatibility was evaluated by haemolysis test, clotting time and platelet adhesion behavior tests. The results show that the haemocompatibility of the alloy could be significantly improved by surface heparinization.
groups, and then the heparin was immobilized on the active TiO2 films through the electrostatic self assembly technology. It is shown that the heparinized films are mainly composed of anatase and rutile with smooth and dense surface. In vitro blood compatibility was evaluated by haemolysis test, clotting time and platelet adhesion behavior tests. The results show that the haemocompatibility of the alloy could be significantly improved by surface heparinization.
Key words: Ti-3Zr-2Sn-3Mo-25Nb alloy; haemocompatibility; heparinization; surface modification; electrostatic self assembly
1 Introduction
Blood coagulation is triggered when a biomaterial is implanted inside a living tissue, or when blood comes in contact with any foreign body. This blood coagulation ultimately results in the formation of a clot. Such clots can stop a medical implant from properly functioning, then endangering the patients’ health [1-3]. There is a continuous need for better blood-compatible surfaces in medical applications [3,4]. It is known that the surface properties (surface roughness, chemical inertia, surface hydrophilic/hydrophobic property, etc) of a biomaterial dominate the interactions between the material and human blood [4-8]. A significant part of current research on haemocompatible materials is focused on the surface modification of already existing materials with proven combinations of properties [4,9-11]. More recently new surface modification techniques have been developed which include immobilization of specific biological molecules on the surface of materials, mostly heparin [12-15]. Heparin has been used to modify biomaterial surface because heparin is a potent anticoagulant agent that interacts strongly with antithrombin III to prevent the formation of fibrin clots [16-19]. But the actual performance of immobilized heparin may vary significantly depending on many factors, including the method of immobilization and the amount and type of heparin, and the anticoagulant action of heparin, namely its interaction with antithrombin, is essentially of an electrostatic nature, although it has a significant degree of specificity [20-23].
A novel near β titanium Ti-3Zr-2Sn-3Mo-25Nb (TLM) alloy exhibits a set of mechanical properties such as high strength, good ductility and a high fatigue limit, which makes it suitable for use in many applications requiring contact with human blood [24,25]. The present work was undertaken to improve the haemocompatibility (especially anticoagulation) of the Ti-3Zr-2Sn-3Mo- 25Nb alloy by using a new surface heparinization method. Firstly, a layer of TiO2 film was prepared on the surface of the Ti-3Zr-2Sn-3Mo-25Nb alloy using the sol-gel method. In order to immobilize heparin on biometals effectively, which are rather inert to this purpose, their surface should be suitably functionalized with proper “anchor” chemical groups of which heparin could possibly be linked, preferably with covalent bonds, in order to remain stable after in vivo implantation [3,7]. An alternative approach utilizing an electrostatic self assembly (ESA) technique was used to modify the surface of biomedical titanium alloys. This technique is based on the consecutive adsorption of polyanions and polycations via electrostatic interactions. In recent years, the ESA technique has emerged as a versatile, inexpensive yet efficient technique to build biologically active surfaces [8,9]. It is a flexible strategy that affords nanometer-scale control of the spatial distribution of the ionized species within the surface films. Electrostatic self-assembled multi-composites make it possible to surface engineer materials by combining two or more desirable properties of biomaterials (e.g., anticoagulation properties) [10,11].
After the surface of the samples was modified using the sol-gel method, the OH- and 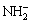 active groups were introduced onto the surface of the TiO2 films using an activation treatment in anhydroxyl and aminated solution. And the multilayer coatings were prepared by the electrostatic self assembly of heparin retaining a significant degree of layered structure. The in-vitro blood compatibility of the Ti-3Zr-2Sn-3Mo-25Nb alloy specimens with and without heparinization treatment was evaluated using hemolysis and blood platelet adhesion tests in addition to clotting time measurement. In this work, the ESA technique was employed to construct the outmost layer with heparin on a near β titanium alloy in order to improve the alloy haemocompatibility.
active groups were introduced onto the surface of the TiO2 films using an activation treatment in anhydroxyl and aminated solution. And the multilayer coatings were prepared by the electrostatic self assembly of heparin retaining a significant degree of layered structure. The in-vitro blood compatibility of the Ti-3Zr-2Sn-3Mo-25Nb alloy specimens with and without heparinization treatment was evaluated using hemolysis and blood platelet adhesion tests in addition to clotting time measurement. In this work, the ESA technique was employed to construct the outmost layer with heparin on a near β titanium alloy in order to improve the alloy haemocompatibility.
2 Experimental
2.1 Materials
Heparin (porcine mucosa, sodium salt, Mr=3×103) and Dextran sulfate (DS) (sodium salt, Mr~5×103) were obtained from Sigma. Discoid implants (diameter 10 mm and height 3 mm) were prepared from Ti-3Zr-2Sn- 3Mo-25Nb alloy bar, which was provided by Northwest Institute for Nonferrous Metal Research, China. The samples were polished using Al2O3 slurry to a surface finish of Ra 0.5 μm. After polishing the samples were first cleaned with acetone and then ultrasonically cleaned in ethanol.
2.2 Preparation of TiO2 films
The TiO2 films were prepared on the surface of the Ti-3Zr-2Sn-3Mo-25Nb alloy samples using a sol-gel technique. Commercially available tetraisopropyl orthotitanate, Ti((CH3)2CHO)4, was dissolved in the absolute ethanol (solution A). Ethyleneglycol monoethylether (C2H5OCH2CH2OH), deionised water, and fuming hydrochloric acid (HCl, 37%) were also dissolved in ethanol (solution B). Solutions A and B were mixed rapidly and stirred for 3 min. The coating sol was composed of EtOH:Ti(OR)4, H2O:Ti(OR)4 and HCl:Ti(OR)4 with molar ratios of 8.2, 1.0 and 0.018, respectively. The gel was aged at 0 °C for 24 h before being used. The coatings were prepared by dipping the Ti-3Zr-2Sn-3Mo-25Nb implants into the sol and then withdrawing them at a speed of 0.20 mm/s. The sol was kept at 0 °C during the dipcoating process. The coated substrates were heat-treated in a small oven at a ramp speed of 3 (°)/min with a dwell time of 1 h at 500 °C. After heat treatment, the coatings were cleaned ultrasonically in acetone for 5 min and in ethanol for 5 min, and finally dried at the ambient temperature.
2.3 Surface heparinization of TiO2 films
The films were treated with an aqueous solution of potassium peroxydisulfate (100g/L) at 75 °C for 6 h dynamically, and finally the samples were washed copiously with hot water and dried in a vacuum. Then the films were submitted to grafting reactions in an aqueous solution with the aid of the ceric ion technique using acrylamide (120 g/L) in nitric acid (0.04 mol/L) and ceric ammonium (0.04 mol/L) solution at 65 °C for 24 h under a stream of nitrogen. Films were washed extensively with NaOH solution followed by hot water.
The activated TiO2 films were cleaned with absolute ethyl alcohol and distilled water and coated by subsequent alternating adsorption of heparin and dextran sulfate (DS) from their respective solutions (1 mg/mL) in 0.015 mol/L citrate buffer (CB), pH 4.0, at room temperature. The first layer of heparin was immobilized by active groups on the supporting surface, and then the following sequence was repeated five times: 1) heparin, 60 min; 2) thorough rinsing with CB; 3) DS, 30 min; 4) rinsing with CB. The assembly consisting of the five heparin-DS double layers was fixed at 55 °C for 60 min with 1% glutaraldehyde in CB, followed by thorough rinsing in CB and then with PBS (pH=7.4), which removed all DS.
2.4 Characterization of films
Phases present in the heparinized TiO2 films were identified using X-ray diffractometry. Graphite monochromator and Cu Kα radiation was also used. The measurements were made for diffraction angle 2θ scanning in the range of 20°-80° with a step of 0.003 (°)/s, the angle of the incident beam was fixed at 2° against the sample surfaces in order to detect the phase composition of the sample surfaces and the measurements were performed with a continuous scanning mode. Surface characteristics of the films were observed by using a scanning electron microscope (JSM-6460, JEOL) and a commercial AFM (Nanoscope IIIa, Digital Instrument Corp., USA), the operating mode of AFM was tapping mode, the scanning rate was 1 Hz and the scanning area was 10 μm×10 μm.
2.5 Haemolysis test
The samples with and without heparinization were immersed in diluted blood solution containing 2% fresh acid citrate dextrose (ACD) human blood and 98% physiological salt solution, and incubated at 37 °C for 1 h. After centrifuging at 700×g for 5 min, the absorbency of the solution was recorded as Dt. Under the same conditions, a solution containing 2% ACD blood and 98% physiological salt solution was used as a negative reference and a solution containing 2% ACD blood and 98% distilled water was used as a positive reference. Their absorbencies were recorded as Dn and Dp, respectively. The haemolysis ratio η of the samples was calculated by
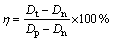
The results presented in this paper represent the statistical average of five measurements on different regions of the sample surface.
2.6 Clotting time measurement
The thromboresistant properties of the films were evaluated using fresh rabbit blood by the kinetic clotting time method. Blood was taken from a rabbit using a plastic syringe, and 0.1 mL of the blood was immediately dropped onto the film specimens. After a predetermined time of 10, 20, 30, 40 and 50 min, specimens were transferred into beakers, each containing 25 mL of distilled water, and incubated for 5 min. The red blood cells that had not been trapped in a thrombus were hemolyzed, and the free hemoglobin was dispersed in water. The concentration of free hemoglobin in the water was colorimetrically measured by monitoring the absorbance at 540 nm using a spectrophotometer. The absorbance values were plotted versus the blood contacting time. Each absorbance value represents the average of five measurements, and the clotting time was derived using optical density curves.
2.7 Platelet adhesion test
In vitro platelet adhesion testing was performed to investigate the quantity, morphology, aggregation and pseudopodium of the adherent platelets. Whole blood obtained from healthy human volunteers was collected in citrate phosphate dextrose (CPD). This was centrifuged at 200×g for 15 min to obtain platelet rich plasma (PRP). The samples were immersed in PRP and incubated at 37 °C for 2 h. The samples were subsequently rinsed with a 0.09 g/L NaCl solution to remove weakly adherent platelets. The adhered platelets were fixed in 2.5% glutaraldehyde solution at room temperature for 12 h, and then dehydrated in a graded ethanol series, and critical point dried, covered using a sputter coater with a layer of gold. Platelet morphology in the case of heparinized and reference materials was examined using a scanning electron microscope operating at 20 kV (JSM 6700, JEOL, USA).
3 Results and discussion
3.1 Crystalline structure and surface morphology
Figure 1 shows the X-ray diffraction patterns associated with the heparinized Ti-3Zr-2Sn-3Mo-25Nb specimens. It can be seen that strong peaks occur for composite films at 2θ=25.3° and 2θ=27.4° which correspond to the (101) and (110) crystal planes. The peaks confirm the presence of the anatase phase and rutile phase in the composite films. Also a strong peak occurs for the TiO2 matrix at 2θ=25.3°, which confirms the presence of only anatase phase in the TiO2 matrix film. And the other anatase and rutile phases can be easily identified with the diffraction peaks being sharp not splitting.
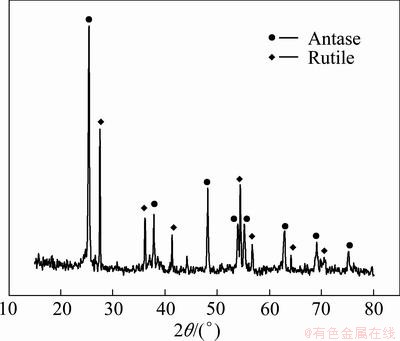
Fig. 1 XRD pattern of sol-gel films after heparinization
It is clear from Figs. 2 (a) and (b) that the TiO2 films cover the whole surface of the Ti-3Zr-2Sn-3Mo-25Nb samples, without cracking and spalling behavior. The surface of the films is smooth, uniform and without the presence of any visible agglomerates, and the surface is much smoother with less cracks present after surface heparinization.
The AFM image (Fig. 2(c)) shows the micrograph of the surface of the heparinized sol-gel films. The surface roughness is around 200 nm which indicates a smooth surface. It is observed that there is a large number of immobilized heparin on the surface of the sol-gel films. The immobilized heparins are globular in nature and they are aggregated as indicated by the arrows in Fig. 2(c). This morphology is related to the physical properties of the outmost heparin component. The high intrinsic chain stiffness and high relative molecular mass for the heparin molecules affect its diffusion ability during the multi-layer build-up process.
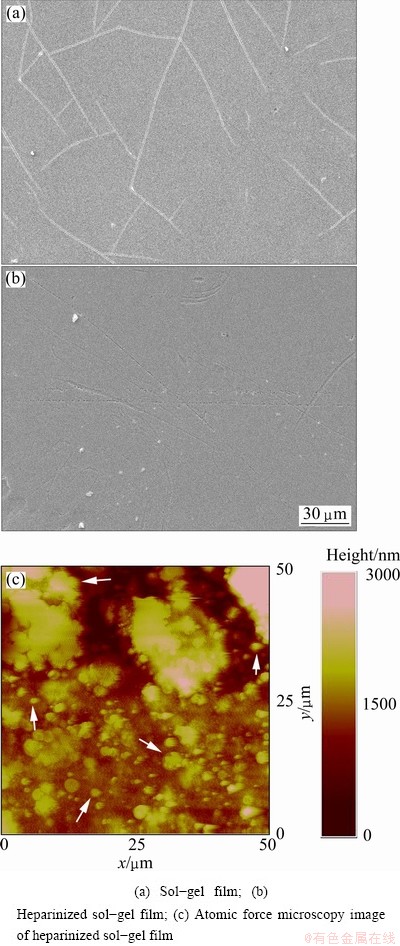
Fig. 2 Micrographs of TiO2 films
3.2 Haemocompatibility
Figure 3 shows the blood clotting profiles for the TiO2 films. The absorbance of the hemolyzed hemoglobin solution varies with time, and the higher the absorbance, the better the thromboresistance. The time at which the absorbance equals 0.01 is generally defined as the clotting time, and a longer clotting time implies better blood compatibility. Figure 3 shows that the clotting time for the heparinized Ti-3Zr-2Sn-3Mo- 25Nb alloy is much longer than that without surface heparinization, illustrating the superior haemo- compatibility of the former. This demonstrates that the anticoagulant properties of heparin were preserved on the Ti-3Zr-2Sn-3Mo-25Nb alloy surface.
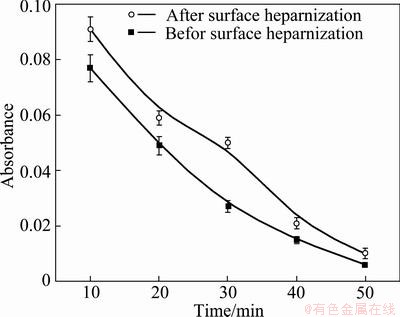
Fig. 3 Absorbance of TLM before and after surface heparinization versus time
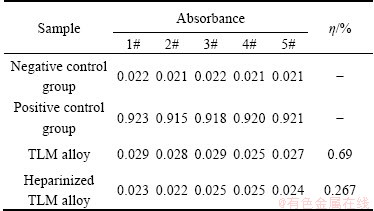
The hemolysis rate of the Ti-3Zr-2Sn-3Mo-25Nb alloy before and after heparinization treatment is shown in Table 1. The average hemolysis rate (η) of the Ti-3Zr-2Sn-3Mo-25Nb alloy before treatment is 0.69%, and the rate after herarinization is 0.267%, which is significantly reduced and could not cause hemolytic reaction in the body. The haemolysis rate is an important factor for characterization of the haemocompatibility. The lower the haemolysis rate, the better the haemocompatibility.
Table 1 Hemolysis rate of TLM alloy before and after surface heparinization
Adhesion and aggregation of platelets play a significant role in thrombus formation, the platelet adhesion test in vitro shows significant differences in the behavior of the platelet adhesion for different Ti-3Zr-2Sn-3Mo-25Nb surfaces, as shown in Fig. 4. On the heparinized surface, only a few platelets adhere and are isolated (Fig. 4(a)). The platelets have a round shape and only slight pseudopodia can be observed (Fig. 4(b)). In comparison, much more platelet adhesion and aggregation is observed on the Ti-3Zr-2Sn-3Mo-25Nb surface (Fig. 4(c)), and pseudopodia are well distributed on the surface (Fig. 4(d)). Thus, the results of in vitro platelet adhesion tests suggest that the surface heparinization not only reduces the number of adherent platelets, but can also deter their activation.

Fig. 4 SEM images for platelet adhesion of TLM samples with (a, b) and without (c, d) heparinization treatment
Haemocompatibility can result from a range of comprehensive factors. In the present research, TiO2 films on the Ti-3Zr-2Sn-3Mo-25Nb alloy is one of the most important factors. The antithrombotic function of the TiO2 films is related to non-stoichiometric rutile structure, wide gap, n-type semiconductor nature and medium level hydrophilicity. In view of adsorption thermodynamics, the adsorption tendency of blood components at the interface between blood and material depends on its adhesion ability. This means that the behavior of adsorbates (such as plasma proteins, blood cells and clotting factors) at the interface can be strongly influenced by the hydrophilic/hydrophobic properties of the surface. Proteins can change their conformation upon adsorption, but suitable hydrophilic surfaces can inhibit the denaturation of adsorbates. Inorganic TiO2 films with wide band gap structures associated with n-type semiconductor can prevent the charge transferring from the protein to the material, because the activation of plasma proteins is related to an electron transfer from the protein to the surface, which is a key mechanism to induce thrombus formation and it is mainly dependent on the net charge of its molecule [5]. The natural TiO2 film on the Ti-3Zr-2Sn-3Mo-25Nb sample is very thin (~40 nm), and it is amorphous. But the thickness of the sol-gel film is over hundreds of nanometers, and with highly crystalline characteristics. In addition, it is believed that the sol-gel TiO2 film is rich in Ti-OH groups that can reduce the contact angle, and the active functional groups of OH- and 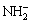 which are introduced onto the surface of the films as hydrophilic groups along with hydrogen bonding which is present on the surface through physical absorption of water. For these reasons the hydrophilicity of the activated films is enhanced, and this is also one of the important reasons why the heperanized films show a decrease of the hemolysis rate. More importantly, the covalent attachment of heparin on the material surface has a profound influence on the behavior of blood coagulation. Heparin is one of the most intensively studied glycosaminoglycans as a result of its anticoagulant properties [13]. Heparinized surfaces show reduced platelet adhesion, reduced haemolysis rate and an increase in the activated partial thromboplastin time, resulting in improved biocompatibility without compromising thrombo-resistant properties. Immobilized heparin, unlike soluble heparin, also inhibits the initial contact activation coagulation enzymes through an antithrombin III (AT-III) mediated pathway, and thus the surface exhibits better anticoagulant properties.
which are introduced onto the surface of the films as hydrophilic groups along with hydrogen bonding which is present on the surface through physical absorption of water. For these reasons the hydrophilicity of the activated films is enhanced, and this is also one of the important reasons why the heperanized films show a decrease of the hemolysis rate. More importantly, the covalent attachment of heparin on the material surface has a profound influence on the behavior of blood coagulation. Heparin is one of the most intensively studied glycosaminoglycans as a result of its anticoagulant properties [13]. Heparinized surfaces show reduced platelet adhesion, reduced haemolysis rate and an increase in the activated partial thromboplastin time, resulting in improved biocompatibility without compromising thrombo-resistant properties. Immobilized heparin, unlike soluble heparin, also inhibits the initial contact activation coagulation enzymes through an antithrombin III (AT-III) mediated pathway, and thus the surface exhibits better anticoagulant properties.
4 Conclusions
1) The heparinized coatings on a near β titanium Ti-3Zr-2Sn-3Mo-25Nb alloy were successfully achieved by preparing a layer of sol-gel TiO2 films and further covalent immobilization of heparin onto these films.
2) The heparinized Ti-3Zr-2Sn-3Mo-25Nb samples exhibit better anticoagulation properties and a lower hemolytic rate than the samples without surface heparinization. The number of adherent platelets on the surface associated with the heparinization treatment is much smaller than that on the Ti-3Zr-2Sn-3Mo-25Nb alloy without heparinization treatment, and the level of aggregation and the amount of pseudopodium are much less than those present on the untreated surface. Therefore, the Ti-3Zr-2Sn-3Mo-25Nb alloy with heparinization shows improved haemocompatibility.
References
[1] PASZENDA Z. Application problems of implants used in interventional cardiology [J]. Information Technologies in Biomedicine, 2008, 47: 15-27.
[2] PENG H T. Thromboelastographic study of biomaterials [J]. Journal of Biomedical Materials Research (Part B): Applied Biomaterials, 2010, 94(2): 469-485.
[3] YANG Zhi-lu, WANG Jin, RIFANG L, MAITZ M F, JING Feng-juan, SUN Hong, NAN Huang. The covalent immobilization of heparin to pulsed-plasma polymeric allylamine films on 316L stainless steel and the resulting effects on hemocompatibility [J]. Biomaterials, 2010, 31(8): 2072-2083.
[4] LIU Xuan-yong, CHU P K, DING Chuan-xian. Surface modification of titanium, titanium alloys, and related materials for biomedical applications [J]. Materials Science and Engineering R, 2004, 47 (3-4): 49-121.
[5] NAN H, YANG P, LENG Y X, CHEN J Y, SUN H, WANG J, WANG G J, DING P D, XI T F, LENG Y. Hemocompatibility of titanium oxide films [J]. Biomaterials, 2003, 24(13): 2177-2187.
[6] GEETHA M, SINGH A K, ASOKAMANI R, GOGIA A K. Ti based biomaterials, the ultimate choice for orthopaedic implants-A review [J]. Progress in Materials Science, 2009, 54(3): 397-425.
[7] MOURTAS S, KASTELLORIZIOS M, KLEPETSANIS P, FARSARI E, AMANATIDES E, MATARAS D, PISTOLLO B R, FAVIA P, SARDELLA E, D’AGOSTINO R, ANTIMISIARIS S G. Covalent immobilization of liposomes on plasma functionalized metallic surfaces [J]. Colloids and Surface B: Biointerfaces, 2011. doi:10.1016/j.colsurfb.2011.01.002.
[8] CAI Kai-yong, RECHTENBACH A, HAO Jian-yuan, BOSSERT J, JANDT K D. Polysaccharide-protein surface modification of titanium via a layer-by-layer technique: characterization and cell behaviour aspects [J]. Biomaterials, 2005, 26(30): 5960-5971.
[9] LUDOVIC R, LAVALLE P, PAYAN E, SHU X Z, PRESTWICH G D, STOLTZ J F, SCHAAF P, VOEGEL J C, PICART C. Layer by layer buildup of polysaccharide films: Physical chemistry and cellular adhesion aspects [J]. Langmuir, 2004, 20(2): 448-458.
[10] CAI Kai-yong, HU Yan, JANDT K D, WANG Yuan-liang. Surface modification of titanium thin film with chitosan via electrostatic self-assembly technique and its influence on osteoblast growth behavior [J]. Journal of Materials Science: Materials in Medicine, 2008, 19(2): 499-506.
[11] ZHA Zheng-bao, MA Yan, YUE Xiu-li, LIU Meng, DAI Zhi-fei. Self-assembled hemocompatible coating on poly (vinyl chloride) surface [J]. Applied Surface Science, 2009, 256(3): 805-814.
[12] JOHNELL M, LARSSON R, SIEGBAHN A. The influence of different heparin surface concentrations and antithrombin-binding capacity on inflammation and coagulation [J]. Biomaterials, 2005, 26(14): 1731-1739.
[13] YAMAGUCHI N, CHAE B S, ZHANG L, KIICK K L, FURST E M. Rheological characterization of polysaccharide-poly(ethylene glycol) star copolymer hydrogels [J]. Biomacromolecules, 2005, 6(4): 1931-1940.
[14] LINHARDT R J, MURUGESAN S, JIN X. Immobilization of heparin: Approaches and applications [J]. Current Topics in Medicinal Chemistry, 2008, 8(2): 80-100.
[15] ZHU Ai-ping, ZHANG Ming, WU Jun, SHEN Jian. Covalent immobilization of chitosan/heparin complex with a photosensitive hetero-bifunctional crosslinking reagent on PLA surface [J]. Biomaterials, 2002, 23(23): 4657-4665.
[16] HUANG L Y, YANG M C. Surface immobilization of chondroitin 6-sulfate/heparin multilayer on stainless steel for developing drug-eluting coronary stents [J]. Colloids and Surfaces B: Biointerfaces, 2008, 61(1): 43-52.
[17] GONG Fei-rong, CHENG Xiao-yan, WANG Shan-feng, ZHAO Yan-chao, GAO Yun, CAI Hai-bo. Heparin-immobilized polymers as non-inflammatory and non-thrombogenic coating materials for arsenic trioxide eluting stents [J]. Acta Biomaterialia, 2010(6): 534-546.
[18] CHEN Hong, CHEN Yang, SHEARDOWN H, BROOK M A. Immobilization of heparin on a silicone surface through a heterobifunctional PEG spacer [J]. Biomaterials, 2005, 26(35): 7418-7424.
[19] SASK K N, MCCLUNG W G, BERRY L R, CHAN A K C, BRASH J L. Immobilization of an antithrombin–heparin complex on gold: Anticoagulant properties and platelet interactions [J]. Acta Biomaterialia, 2011, doi:10.1016/j.actbio.2011.01.031.
[20] CHUANG T W, LIN D T, LIN F H. Immobilization of NaIO4-treated heparin on PEG-modified 316L SS surface for high anti-thrombin-III binding [J]. Journal of Biomedical Materials Research Part A, 2008, 86(3): 648-661.
[21] MICHANETZIS G P A, KATSALA N, MISSIRLIS Y F. Comparison of haemocompatibility improvement of four polymeric biomaterials by two heparinization techniques [J]. Biomaterials, 2003, 24(4): 677-688.
[22] YU Sen, YU Zhen-tao, WANG G, DARGUSCH M S, ZHANG Ming-hua. Evaluation of haemocompatibility of TLM titanium alloy with surface heparinization [J]. Rare Metal Materials and Engineering, 2009, 38(3): 384-388.
[23] CHRISTENSEN K, LARSSON R, EMANUELSSON H, ELGUE G, LARSSON A. Improved blood compatibility of a stent graft by combining heparin coating and abciximab [J]. Thrombosis Research, 2005, 115(3): 245-253.
[24] YU Zhen-tao, WANG G, MA Xi-qun, DARGUSCH M S, HAN Jian-ye, YU Sen. Development of biomedical near β titanium alloys [J]. Materials Science Forum, 2009, 618-619: 303-306.
[25] YU Zhen-tao, WANG G, MA Xi-qun, ZHANG Ya-feng, DARGUSCH M S. Shape memory characteristics of a near β titanium alloy [J]. Materials Science and Engineering A, 2009, 513-514: 233-238.
余 森1,于振涛1,韩建业1, WANG Gui2, 牛金龙1, Matthew S. Dargusch2
1. 西北有色金属研究院,西安 710016;
2. CAST Cooperative Research Centre, School of Mechanical and Mining Engineering, The University of Queensland, Brisbane QLD 4072, Australia
摘 要:为了提高新型近β钛合金Ti-3Zr-2Sn-3Mo-25Nb(TLM)的抗凝血性能,先通过优化溶胶-凝胶技术在TLM合金试样表面制备出一层致密、均匀的TiO2薄膜,再结合表面官能化处理和静电层自组装技术,在TiO2薄膜表面构建一个多层肝素功能涂层。通过XRD分析改性处理后TLM合金表面涂层的相组成,借助 SEM、AFM研究功能涂层的表面形貌和微观特征,通过动态凝血时间、溶血率、血小板粘附实验对TLM合金表面改性前后的抗凝血性能和血液相容性进行评估。结果表明,经表面肝素化修饰处理后,TLM合金试样表面光滑、平整,同时材料表面的动态凝血时间延长,溶血率明显降低,试样表面粘附的血小板很少且没有被明显激活,TLM合金的抗凝血性能和血液相容性均得到了明显的改善。
关键词:Ti-3Zr-2Sn-3Mo-25Nb钛合金;血液相容性;肝素化;表面改性; 静电自组装
(Edited by LI Xiang-qun)
Foundation item: Project (31100693/C100302) supported by the National Natural Science Foundation of China; Project (31011120049) supported by the Australia-China Special Fund, International Science Linkages Program co-supported by the Department of Innovation, Industry, Science and Research of Australia, and the Ministry of Science and Technology and National Science Foundation of China; Project (2010ZDKG-96) supported by the Major Subject of “13115” Programs of Shaan’xi Province, China; Project (2012CB619102) supported by the National Basic Research Program of China
Corresponding author: YU Sen; Tel: +86-29-86231084; E-mail: ninbrc@gmail.com
DOI: 10.1016/S1003-6326(11)61569-0

