Effects of temperatures and pH values on rheological properties of cemented paste backfill
来源期刊:中南大学学报(英文版)2021年第6期
论文作者:陈秋松 张钦礼 李奕腾 刘一锴 冯岩 王道林
文章页码:1707 - 1723
Key words:cemented paste backfill (CPB); rheological properties; temperatures; pH values; cement hydration; microscopic analysis
Abstract: In this study, different influence mechanisms associated with temperatures and pH values were investigated through cemented paste backfill (CPB) systems. CPB samples were prepared with temperatures ranging from 10 to 50 °C in 10 °C increments and pH values of 3, 7, and 13. Then, the CPB mixture were subjected to rheological tests, thermogravimetric analysis (TG), derivative thermogravimetry analysis (DTG), Fourier-transform infrared spectroscopy (FT-IR), and scanning electron microscopy (SEM). Results demonstrated that the temperatures had significant effects on the rheological properties of CPB, whereas the effects of pH values were relatively unapparent. Higher temperatures (over 20 °C) were prone to bring higher shear stress, yield stress, and apparent viscosity with the same pH value condition. However, an overly high temperature (50 °C) cannot raise the apparent viscosity. Non-neutral conditions, for pH values of 3 and 13, could strengthen the shear stress and apparent viscosity at the same temperature. Two different yield stress curves could be discovered by uprising pH values, which also led to apparent viscosity of two various curves under the same temperatures (under 50 °C). Microscopically, rheological properties of CPB were affected by temperatures and pH values which enhanced or reduced the cement hydration procedures, rates, products and space structures.
Cite this article as: ZHANG Qin-li, LI Yi-teng, CHEN Qiu-song, LIU Yi-kai, FENG Yan, WANG Dao-lin. Effects of temperatures and pH values on rheological properties of cemented paste backfill [J]. Journal of Central South University, 2021, 28(6): 1707-1723. DOI: https://doi.org/10.1007/s11771-021-4728-4.

J. Cent. South Univ. (2021) 28: 1707-1723
DOI: https://doi.org/10.1007/s11771-021-4728-4
ZHANG Qin-li(张钦礼)1, LI Yi-teng(李奕腾)1, CHEN Qiu-song(陈秋松)1, 2,LIU Yi-kai(刘一锴)3, FENG Yan(冯岩)1, WANG Dao-lin(王道林)1
1. School of Resources and Safety Engineering, Central South University, Changsha 410083, China;
2. Sinosteel Maanshan Institute of Mining Research Company Limited, Maanshan 243000, China;
3. Department of Geoscience, University of Padova, Via Gradenigo 6, I-35131 Padova, Italy
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract: In this study, different influence mechanisms associated with temperatures and pH values were investigated through cemented paste backfill (CPB) systems. CPB samples were prepared with temperatures ranging from 10 to 50 °C in 10 °C increments and pH values of 3, 7, and 13. Then, the CPB mixture were subjected to rheological tests, thermogravimetric analysis (TG), derivative thermogravimetry analysis (DTG), Fourier-transform infrared spectroscopy (FT-IR), and scanning electron microscopy (SEM). Results demonstrated that the temperatures had significant effects on the rheological properties of CPB, whereas the effects of pH values were relatively unapparent. Higher temperatures (over 20 °C) were prone to bring higher shear stress, yield stress, and apparent viscosity with the same pH value condition. However, an overly high temperature (50 °C) cannot raise the apparent viscosity. Non-neutral conditions, for pH values of 3 and 13, could strengthen the shear stress and apparent viscosity at the same temperature. Two different yield stress curves could be discovered by uprising pH values, which also led to apparent viscosity of two various curves under the same temperatures (under 50 °C). Microscopically, rheological properties of CPB were affected by temperatures and pH values which enhanced or reduced the cement hydration procedures, rates, products and space structures.
Key words: cemented paste backfill (CPB); rheological properties; temperatures; pH values; cement hydration; microscopic analysis
Cite this article as: ZHANG Qin-li, LI Yi-teng, CHEN Qiu-song, LIU Yi-kai, FENG Yan, WANG Dao-lin. Effects of temperatures and pH values on rheological properties of cemented paste backfill [J]. Journal of Central South University, 2021, 28(6): 1707-1723. DOI: https://doi.org/10.1007/s11771-021-4728-4.
1 Introduction
Deep mining engineering has been inspiringly progressed, owing to its fascinating contribution to the modern industry. However, various problems have arisen in recent years, such as high working temperatures, high rock pressure, and the high probability of rocks bursting [1, 2]. Besides, environmental problems relating to the excavation operation and tailings disposal, for instance, the presence of heavy metals in leachate, can threaten human health and have a greater possibility of surface and groundwater pollution [3, 4]. Therefore, the long-term safe disposal of solidified hazardous waste is a critical request for keeping the surroundings more secure for the coming generations. Accordingly, cemented paste backfill (CPB) technology is recognized as an optimum method for addressing the above problems. It transfers the produced solid waste, such as tailings, into underground goaf by mixing an experimented proportion of cementitious material and water. This attempt not only effectively reduce the opportunities of rock bursts but also reduce the secondary environmental pollution and the risk of failure of tailings dams [5-8].
Many researchers concerning CPB technology focused on its early-age mechanical properties, pores structures, and long-term durability [9-11]. Previous studies have confirmed that rheological parameters, including yield stress and apparent viscosity, are significant factors when constructing the CPB pipeline transportation system [12-15], as it guarantees the transport of CPB within the stopes.
Generally, mining companies are prone to use tap water for CPB mixing, which is originated from water plants. However, with the impacts of the mining procedures, local climates, and long transportation, transported water properties cannot remain stable. On the other hand, wastewater from ore-dressing processes was also used to mix CPB, with the increasing concerns about environmental protection and waste recycling [16]. However, as mentioned above, the wastewater itself also lacks a stable pH value and temperature to guarantee CPB performance due to different mining processes, mineral processing, and local hydrogeological conditions.
Previous research indicates that temperature significantly affects the rheological properties of CPB. As the temperature increases (under 60 °C), cement hydration progress can be accelerated, and more hydration products are produced; that is, the interactive frictional resistance of the cemented particle materials in CPB may give rise to high viscosity and shear stress [17, 18]. On the other hand, the flow performance of CPB becomes better due to the volume expansion coefficient of CPB exponentially increases. Meanwhile, tailings are pressed, and the interaction force of the particles increases, which leads to high yield stress [19]. Moreover, the spatial information of the adsorbed polymer chains in CPB might be changed, and electrosteric potential of particles might cause a lower degree of thixotropy [20]. For further study, some scholars investigated the additions in CPB rheology considering the temperature [21, 22]. High-range water-reducing admixtures added in CPB may retard the cement hydration, which incorporates the side chain of the polymer as the temperature increases, and therefore, leading to higher initial yield stress [23].
With regard to pH values, when pH>12, the zeta potential of fresh CPB increases because the particle surface is adsorbed by a mass of poorly-hydrated ions (Cs+, K+), which gives rise to agglomeration and increase in the yield stress. That is, the ions might minimize the free energy by occupying the neighbouring particles’ positions [24-26]. With the same pH value, the vast majority of soluble Ca2+ from the binder are released into CPB during the initial stage [27]. Meanwhile, it is the reason why hydroxypropyl-methyl cellulose ether molecules can enhance the viscosity-enhancing effect by combining these free Ca2+ [28]. When pH value rises above 10, and the Ca2+ concentration exceeds 1 mmol/L, CPB in fresh enters the “dormant” state and undergoes gelation owing to refinement of the pore structure, resulting in high yield stress [29].
When pH<7, generally, strong/weak acidic solutions may be deactivated by enough alkalinity produced by CPB. A few scholars studied the acid reagents on the CPB rheology. However, it is mainly focused on superplasticizers (SP). It is reported that the fluidity of CPB is decreased meanwhile with an increase in viscosity value by a higher number of lignosulphonates (pH 6.87). That is to say, lignosulphonates can accelerate the formation of ettringite [30]. Whereas, polycarboxylates may prevent the alite dissolution in CPB and perform a better viscosity [31, 32]. Nevertheless, low pH values on CPB rheology, especially low pH values solutions for CPB mixing, remains sparsely researched.
Although numerous publications have studied the rheological properties of CPB, few papers have considered the influence of temperatures and pH values, as well as the related microscopic mechanisms. Therefore, in this study, the physical and chemical properties of raw materials were firstly tested. Then, the rheological shear tests of CPB at different temperatures and pH values were conducted. Subsequently, the physicochemical properties and chemical composition of CPB were investigated through TG&DTG, FT-IR, and SEM.
2 Materials and methods
2.1 Raw materials
2.1.1 Tailings and binder
The samples used in this study were obtained from a lead-zinc mine in Gansu province, China, after the flotation processing of ores. The density of the tailings was determined as 2.70 t/m3, by a density tester (ET-320R, Etnaln, China). The permeability coefficient was 2.06×105 cm/s via a permeameter (TST-70, North Engineering, China), and the specific surface area was 1.32 m2/g, measured via a Malvern Mastersizer 2000 laser particle size analyzer. The particle size distribution of the tailings was measured by the same laser particle size analyzer, shown in Figure 1. In this experiment, half of the tailings had a particle size less than 21 μm, 90% of the particle diameters were less than 223 μm, and the volume-based particle size was defined as coarse-grained [33, 34].
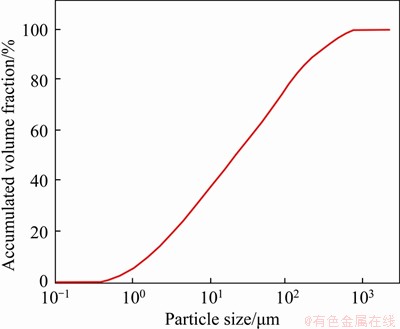
Figure 1 Particle size distribution of tailings
The X-ray diffraction pattern (Figure 2) and the mineral composition (Table 1) demonstrated that the main mineral compositions of tailings are CaCO3, FeCO3, SiO2, and Ca(Mg, Fe)(CO3)2. The workability and rheology performance of CPB in the pipeline are affected by these trigonal crystal structures, bringing a higher degree of wear to pipes [35]. The chemical composition of the tailings was obtained by X-ray fluorescence spectrometry (S4 Pioneer, Bruker, Germany), as shown in Table 2. The main oxide components of these tailings are SiO2 (29.55%), CaO (25.52%), and Fe2O3 (23.73%).
The binder used in this study was P·O 42.5 ordinary Portland cement supplied by Sinoma Cement Co., Ltd., China (GB175—2007). Its chemical composition is presented in Table 3.
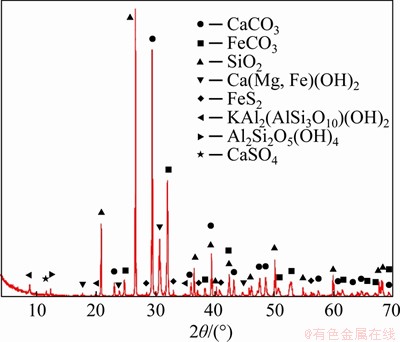
Figure 2 XRD pattern of tailings
Table 1 Mineral composition quantitative analysis of tailings by XRD analysis (wt%)

Table 2 Chemical composition of tailings by XRF analysis (wt%)
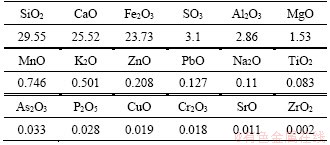
Table 3 Chemical composition of cement by XRF analysis (wt%)
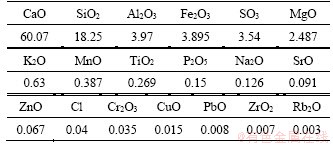
2.1.2 Water and pH-adjusting solution
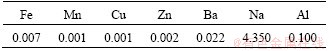
Tap water with a pH value of 7 was used to mix the CPB. To exclude external liquid substances, heavy metal ions and sulfate ions were recorded in Table 4. The tap water meets the standard of drinking water in China (GB5749—2006), containing minimal quantities of metal elements.
Table 4 Substances in tap water (mg/L) [36]
Diluted hydrochloric acid and sodium hydroxide solutions were respectively applied to satisfy the pH values demands in this study. The customized acid reagent was a 0.10 mol/L diluted hydrochloric acid standard solution with a pH value of 3. And alkaline reagent was a 0.10 mol/L sodium hydroxide standard solution with a pH value of 13. The pH values were conducted at 3, 7 and 13. The pH values of 3 and 13 were used as representative of water from ore-dressing plants or nearby water sources.
2.2 Mix proportions and temperature control
According to the filling standard (GB—50771—2012), the mass content of CPB was set at 75%, and the control group did not have any cement added to it. The cement-tailings ratio was conducted at 0.25 due to the subsequent experiments’ practical experience and accuracy according to the mentioned filling standard. The samples’ parameters are presented in Table 5.
Table 5 Sample parameters of rheological tests
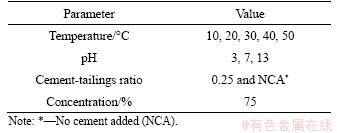
Considering the complex regional environments, the water temperatures applied in industrial systems usually range from 20 to 40 °C (GB—50771—2012). An excessive working temperature has a relatively large impact on the field operation, material consumption, personal safety, and properties of the cement [37]. What’s more, in cold regions with high latitude and altitude, water-body only with 10 °C was severely accounted for CPB generation [38]. Therefore, the crossover experiments were accordingly conducted with the temperatures controlled at 10, 20, 30, 40 and 50 °C. The pH values were conducted as abovementioned, at 3, 7 and 13. The pH values of 3 and 13 were used as representative of water from ore-dressing plants or nearby water sources, which could be polluted by highly concentrated leachates and wastewater [39]. The acid environment, from adding dilute hydrochloric acid, not only increases the instability of formed hydration products [40, 41], but also leads to the dissolution of the cement particles [42]. The addition of sodium hydroxide solution accelerates the early hydration of cement due to the calcium silicate phase and precipitation of the hydrated phase [43].
In rheological tests, the results of samples (including NCA) at 10, 30, and 50 °C with the pH value of 7, samples at 20 °C with the pH value of 3, 7, 13 were collected respectively for discussion. The low temperature was represented by 10 °C while the high temperature was 50 °C. For better comparison, 30 °C was chosen to represent the normal temperature. Moreover, 20 °C and the pH value of 7 were to simulate the normal conditions.
The rheological tests were performed through a HAAKE viscometer rotational rheometer (Thermo Fisher Scientific, USA). The proportional torque and set speed are measured to calculate real-time apparent viscosity, shear stress, and shear rate [44]. The used rheometer has a single-axis six-blade rotating vane with a blade height of 6 cm and a blade width of 2 cm, which can effectively control the slippage behavior and reflect the rheological properties more accurately [45-47]. In order to guarantee a relatively constant temperature, two thermostats (Yongguangming SHHW21.600) were used, one for the underway temperature during experiments, the other for bathing the water solution at a specific temperature to control the initial temperature before experiment conducting.
2.3 Experimental methods
After explored the chemical and physical properties of tailings and cement, CPB mixture were configured at different pH values and temperatures, followed by rheological tests, TG&DTG, FT-IR, and SEM analysis.
2.3.1 Rheological tests
Two thermostat bath boxes were preliminarily set up at the required temperature for heating the HCl solution, NaOH solution, and tap water. The pH value of tap water was adjusted to the desired values by adding the HCl solution or NaOH solution. The desired solutions were maintained at the specified temperature for 30 min to stabilize the temperature state. After placing both dry tailings and binder into a 250 mL beaker and mixing well, it was slowly poured into the thermostat’s aqueous solution whilst being slowly stirred with a glass rod. Then the materials was stirred for 5 min. The tailings and binder were mixed at configured temperatures and pH values. For reducing the CPB mixture evaporation, the beaker was sealed by plastic wrap.
According to the actual in-situ application [34], the general conveying speed of CPB is 1.5 m/s. For most mines in China, the backfill pipes are predominantly over 2 km. Therefore, it takes 22.2 min for CPB to be transported to the goal, and hence 23 min is set as the total hydration time. Therefore, with the initial stirring of 5 min, and the subsequent pre-shearing of 1 min, the hydration time before subjected to rheological tests was 17 min. Then, to eliminate the initial error of the experimental instrument, minimize the thixotropy of cement, and reduce the self-settlement [48, 49], a 1 min pre-shear test with a 40 s-1 shear rate was accordingly conducted.
Subsequently, the vane was slowly immersed and the immersion method followed is that proposed by PANCHAL et al [35]. The shear rate control was gradually increased from 0 to 30 s-1 in 180 s shear time, with the temperature control respectively set according to the initial temperature. The procedures were repeated three times in each set. After the rheological tests, the spatula was used to transfer adequate CPB mixture in beakers containing anhydrous ethanol for stopping the hydration and further characterization [50]
2.3.2 TG&DTG and microscopic analyses
The collected CPB was firstly dried at 45 °C for 3 h before the experiment [51]. For TG&DTG experiments, a Q600 synchronous thermal analyzer from TA Instruments was used. The samples were heated from 40 to 1000 °C with an ascent rate of 10 °C/min, using the argon atmosphere and alumina tray. The FT-IR transmission [52] was carried out through a Thermo Fisher Nicolet Is10, with a 400 to 4000 cm-1 infrared transmission scanning range, measured via a KBr tableting method [53]. The samples were mixed with KBr and uniformly ground and subsequently vacuum pressed into sheets. The SEM instrument used was the JEOL JSM-7900F, with an acceleration voltage of 20 kV [54]. The dried samples were sheeted by Pt glue and carefully placed onto carbon tape attached to the sample holder.
3 Results and discussion
3.1 Rheological tests results
As shown in Figure 3(a), with the increase in shear rate, the shear stress curves of the temperature control group show two different trends: monotonous pseudoplastic fluid (CPB at 10 °C and NCA at 30 °C) and pseudoplastic fluid with overshooting stage (CPB at 30 and 50 °C). As a general rule, the slope curve presents the stress overshoot behavior, especially at high temperatures. It is attributed to the changes in cement particles according to Ref. [55], when the shear rate is at or below 40 s-1, the CPB at 30 and 50 °C generate a stress overshoot stage until the rheological behaviors are stabilized. Then, the rheological curves are converted into typical Bingham fluids. To predict the rheological behaviors, the Herschel-Bulkley function and orthogonal regression iterative algorithm are applied to model the results. Figure 3(a) shows that only the NCA at 30°C and CPB at 10 °C have the appropriate fitting functions by comparing R2. Besides, the shear stress of NCA at 30 °C is generally lower than the CPB curves. As plotted in Figure 3(b), with the increase of shear rate, the apparent viscosity of the temperature control group initially shows shear thickening, followed by thinning.
Concerning the pH value control group in Figure 3(c), all the shear stresses gradually increase with the shear rate, but the overshoot stage does not demonstrate. The calculated Herschel-Bulkley models suggest that all the samples perform significant yielding pseudoplastic behaviors. Among the three CPB samples, the shear stress of CPB at a pH value of 7 is moderate at 65 Pa. Whereas, the shear stress of the samples with pH values of 3 and 13 is relatively high, near to 80 Pa.
As shown in Figure 3(d), as the shear rate raises, the apparent viscosity of the experimental groups initially shows shear thickening and, subsequently, shear-thinning behavior. Additionally, the CPB apparent viscosity curves of pH value of 3 and 13 are approximately the same. Meanwhile, both samples have the highest initial apparent consistency of the experimental groups, near to 130 and 120 Pa·s.
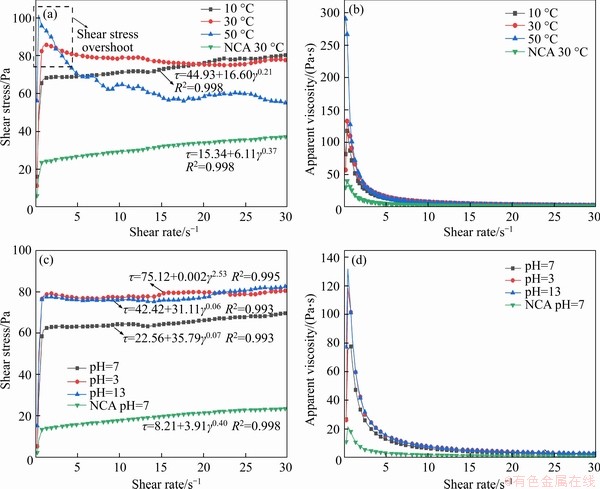
Figure 3 (a) Shear stress of CPB changes with shear rate at different temperatures; (b) Apparent viscosity of CPB changes with shear rate at different temperatures; (c) Shear stress changes of CPB with shear rate at different pH values;(d) Apparent viscosity of CPB changes with shear rate at different pH values
The yield stress and apparent viscosity of the experimental groups at 10 to 50 °C, pH values of 3, 7, 13 are integrated at a shear rate of 30 s-1, as shown in Figure 4.
As shown in Figure 4(a), the yield stress of the temperature control group decreases at 10 and 20 °C. Then the yield stress increases with the rise of temperature. As shown in Figure 4(b), for the pH value control group, the effect on pH values can be divided into two major parts. The yield stress increases as the pH values increase, representing the CPB at 10, 20 and 30 °C. Other parts are “hillside” type yield stress represented by 40 and 50 °C. The main difference in these two parts is that the yield stress decreases when the pH values change from 7 to 13.
Compared with the yield stress trend,shear-thinning viscosity does not demonstrate a specific rule. As the temperature increases, the apparent viscosity of the CPB in the three groups generally shows a decreasing trend, as shown in Figure 4(c). With regard to the influence of pH values, the CPB apparent viscosity at different temperatures demonstrates a “valley” trend with increasing pH value. However, CPB at 50 °C demonstrates the opposite behavior, and is most affected at higher temperatures. Furthermore, the apparent viscosity still follows the rule of higher temperature corresponding to lower apparent viscosity, as shown in Figure 4(d).
According to the results of rheological tests following by Figures 3 and 4. TG&DTG and microscopic analyses were conducted to investigate the reasons which caused the following phenomenons:
1) For temperature, higher temperatures (over 20 °C) may cause higher shear stress in the same pH value condition, so as to the higher yield stress.
2) Higher temperatures also may lead to higher apparent viscosity in the same pH value condition. Moreover, too high temperatures (50 °C) may increase the apparent viscosity.
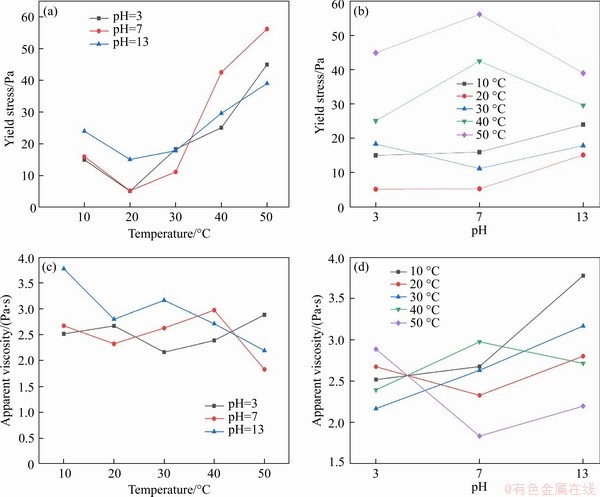
Figure 4 (a) Yield stress trend line at different temperatures; (b) Yield stress trend line at different pH values; (c) Apparent viscosity trend line at different temperatures; (d) Apparent viscosity trend line at different pH values
3) For pH values, non-neutral condition, especially pH value of 3 and 13 may lead to higher shear stress and apparent viscosity in the same temperature.
4) pH values of 3 and 13 may also lead to two different types of yield stress curves and apparent viscosity curves at the same temperature.
3.2 TG&DTG
It can be seen that all the CPB mixture with cement have three main weight-loss areas: 90-110 °C, 370-530 °C and 580-770 °C. The range of 90–110 °C is the predominant C-S-H dehydration decomposition zone. However, owing to the short hydration time, the C-S-H gel is not speedily formatted [56], it is hard to accurately distinguish the weight loss of C-S-H (Figure 5(a)). Despite the short curing age, the DTG curves confirm that C-S-H gel has been formed in all three temperature-controlled CPB samples, which was also suggested by ROSHANI et al [57]. Meanwhile, it is observed that the NCA at 30 °C also peaked at 90-110 °C. The DTG curve integral reflects the relative weight loss of the corresponding substances, by calculation, and hence the DTG curve peak integrals of the three groups of CPB from 90 to 110 °C are greater than the peak integral of NCA at 30 °C. This may be due to the transformation of dihydrate gypsum from the tailings.
370-530 °C is the predominant decalcification zone of calcium hydroxide. The highest concentration of calcium hydroxide of the three CPB groups is the samples at 30 °C, while the 10 °C minimum is probably due to the too low temperature to raise hydration reaction rate, which reduces the calcium hydroxide content. Moreover, the higher temperature prematurely saturates the calcium hydroxide forms. Most significantly, the rate at which the cement hydrates to form calcium hydroxide is slowed down. It can be seen from Figure 5(a) that the calcium hydroxide content of the control group NCA at 30 °C is approximately 9%. When adding cement to form CPB, the calcium hydroxide content is reduced to 7%, probably owing to CaO in the tailings involved in a hydration reaction. According to Figure 5(b), except for the content of calcium hydroxide in each temperature group in the range of 370-530 °C, a small peak appears in NCA at 30 °C when the temperature reaches 360 °C, which is speculated to be the weight loss caused by the decomposition of a small amount of aluminosilicate. Owing to the slow hydration rate at 10 °C, the aluminosilicate hydration products could not be generated within 25 min, while at 50 °C, a small number of hydration products were decomposed prematurely owing to the higher temperature [58]. Moreover, according to the XRD pattern of tailings, the small peak may be caused by halloysite in tailings [59].
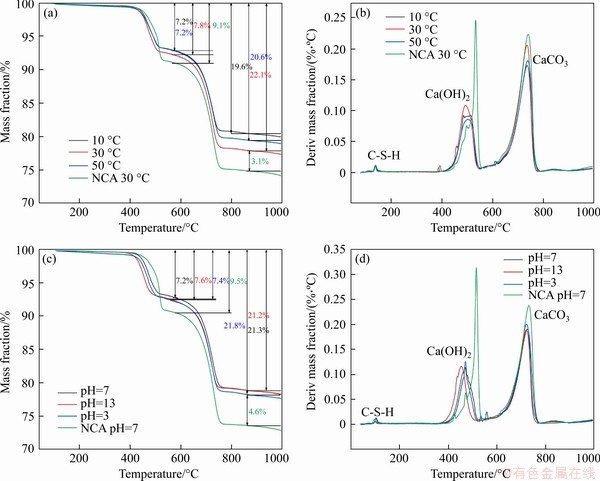
Figure 5 (a) TG curves of temperature control group; (b) DTG curves of temperature control group; (c) TG curves of pH values control group; (d) DTG curves of pH values control group
580-770 °C is a well-known calcium carbonate decomposition weight loss zone. The CaCO3 content is proportional to the content of calcium hydroxide in each temperature group. CaCO3 is mainly caused by the dehydroxylation of calcium hydroxide and the natural carbonisation of cement. Part of CaCO3 took part in the cement hydration and led to the decreasing of CaCO3 [60].
For the pH value control group, the quantity of C-S-H gel produced by the three sets of CPB samples with different pH values is approximately the same as in the dehydration zone of mainly C-S-H gel.
In the decomposition weight loss area of calcium hydroxide, it is known from Figure 5(c) that the CPB sample with pH value of 13 had the highest calcium hydroxide content owing to the strongly alkaline and shorter induction period. Similarly, a higher rate of hydration led to this group containing the most hydrogen [61], at approximately 7.6%. This is followed by the CPB sample with pH value of 3, with a calcium hydroxide content of approximately 7.4%. Since an HCl solution is used to obtain pH value of 3, during cement hydration,low-concentration chloride ions gradually replace hydroxide in the hydration products of Afm [62], so that calcium hydroxide is more than the CPB sample with pH value of 7, which includes tap water. The above conjecture can be verified from the DTG curve.
In the decomposition weight loss zone of calcium carbonate, the CPB sample with pH value of 13 contains the most calcium hydroxide. However, it does not have the most CaCO3 (approximately 13.6%), which is the least among the pH value groups. It is speculated that there are three main points of interest. On the one hand, a high concentration of hydroxide hinders the dissolution of calcium hydroxide in a strongly alkaline environment. The low calcium ion concentration produces less CaCO3 via the cement carbonisation process, compared to the other groups [63]. On the other hand, during the uniform carbonisation time, carbon dioxide additionally reacts with the free hydroxide in the CPB sample under alkaline conditions, and thus, the calcium hydroxide is less carbonised. Finally, CaCO3 formed by carbonisation is relatively stable in an alkaline environment, covering the surface of calcium hydroxide and further preventing the carbonisation of calcium hydroxide [64].
In short, TG&DTG demonstrates that C-S-H gel, calcium hydroxide were produced in CPBs which were formed in a short time and calcium carbonate decomposition was confirmed. Moreover, by observing the quantity of these cement hydration products, the temperatures and non-neutral conditions do affect the quantity of these products to some extent. Therefore, higher temperatures [57] and pH values of 3 and 13 lead to high shear stress, yield stress and apparent viscosity [29].
3.3 FT-IR
The spectral range and distribution of the temperature control group and the pH value control group are shown in Table 6. The FTIR characteristic region and FT-IR fingerprint region of the tested samples are shown in Figure 6.
As shown in Figure 6(b), from the perspective of spectral range and distribution, multiple peaks in the fingerprint region are interleaved within the range 400-1000 cm-1. 417-518 cm-1 corresponds to multiple vibrations of the single bonds of Al—O and Si—O in the CPB mixture, which are not observed in NCA samples. According to the spectral distribution, this range is attributed to the stretching motion of O—Si—O, which is speculated to be caused by the change of C/S ratio in C-S-H after the addition of cement [70].
798 cm-1 is the predominant Si—O vibration zone of the CPB group, which belongs to C-S-HQ1. Due to the short hydration time, all the experimental groups have great transmittances. But, the CPB with greater temperatures produced more C-S-H gel. Moreover, the transmittances at 30 and 50 °C are lower than those of NCA, proving that NCA does not produce C-S-HQ1 owing to the vibration of Si—O, which may belong to dihydrate gypsum and hemihydrate gypsum. This can be further verified by TG&DTG.
Table 6 Assignments to peaks observed in Figure 6
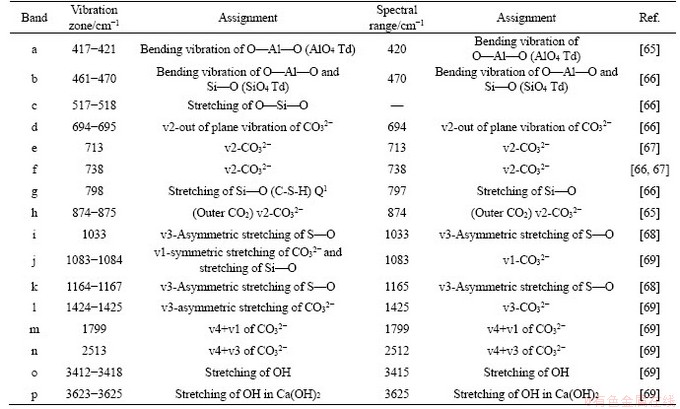
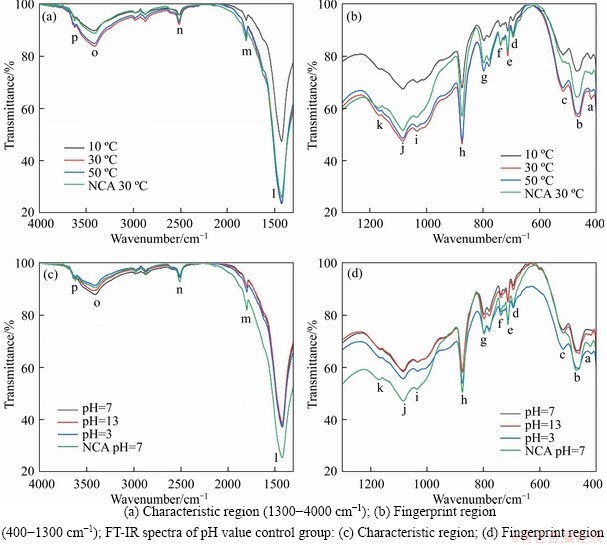
Figure 6 FT-IR spectra of temperature control group:
The peaks at 694-875 cm-1 are predominantly the secondary vibration zone of C—O. The main representative is CaCO3, which is derived from the CPB itself and cement carbonisation. The highest transmittance of CPB at 10 °C indicates that the main CaCO3 content and concentration is low. The lowest CPB transmittance at 30 and 50 °C mean that they have greater contents of CaCO3, which is consistent with the TG&DTG analysis. More significantly, 1083-1084 cm-1 is also a vibration bond of C—O, corresponding to the symmetrical vibration of the C—O single bond.
1033-1167 cm-1 is predominantly the S—O vibration zone, mostly derived from gypsum in the cement and tailings. The content is highest at 30 °C and lowest at 10 °C, depending on the transmittance. Meanwhile, it is proposed that the temperature influences the reaction rate, which can be verified from TG&DTG.
Beyond 1300 cm-1 is the FT-IR characteristic region, as shown in Figure 6(a). 1424-1425 cm-1, 1799 and 2513 cm-1 still belong to C—O vibration zone. Among them, 1424-1425 cm-1 reaches the lowest transmittance of the entire spectrum. It thus predominantly represents the maximum content of CaCO3. Furthermore, this can be verified from the maximum peak value from the TG&DTG results.
3412-3418 cm-1 is attributed to the stretching vibration of OH, mainly from water between the molecules. Consistent with other spectra, the CPB at 30 °C still has the lowest transmission. Furthermore, 3623-3625 cm-1 is still attributed to the stretching vibration of OH but it is mainly attributed to the hydroxyl function of Ca(OH)2. According to the TG&DTG analysis, the Ca (OH)2 content of the CPB is approximately 7% and the lower content corresponds to a higher transmittance.
In general, the CPB at 30 °C has the lowest transmittance in the whole spectrum, followed by 50 and 10 °C. This indicates that the CPB at 30 °C promotes the maximisation of a hydration reaction due to the appropriate temperature, while the low temperature and high temperature limit the further development of cement hydration in terms of reaction rate, chemical composition, and saturation, confirming the speculation of TG&DTG.
As shown in Figures 6(c) and (d), the FT-IR characteristic region and FT-IR fingerprint region of the pH value control group correspond.
In the fingerprint region, the spectral range corresponding to the chemical bond is approximately the same as that of the temperature control group. It is worth noting that the transmittance in the fingerprint region is ranked from low to high primarily according to pH value of 3, 13, and 7. Combined with TG&DTG, it is believed that CPB with pH value of 3 has the highest content and concentration of hardened substances. Furthermore, according to DONATELLO et al [71], an increase in the absorption zone of the CPB with pH value of 3 in the a–c zone, g zone, and j zone is mainly attributed to the enrichment of the silicate acid-insoluble crystalline phase. Moreover, the increased zone in the 600-680 cm-1 region corresponds to the Al—O bond environment, which is probably related to the decomposition products of ettringite [72].
In the characteristic region, the CPB samples with pH value of 7 and pH value of 13 gradually dominate low transmittance, after the “n” region. The regions labelled “o” and “p” belong to the vibration zone of OH and the CPB sample with pH value of 3 demonstrates high transmittance in the absence of OH.
In general, except that the CPB sample with pH value of 3 presents a higher transmittance in the OH vibration zone, the rest of the spectrum predominantly presents a lower transmittance than the CPB samples with the other two groups of pH values. Hence, the CPB sample with pH value of 3 has a comparative advantage in cement hydration. In addition, due to the control of NCA, the three groups with cement can significantly distinguish the control group.
FT-IR was a method to verify the results of TG&DTG. The phenomenon of higher temperatures and non-neutral conditions causing higher rheological parameters values could be verified by FT-IR as well [73, 74]. TG&DTG discussed the reasons on the whole while FT-IR studied the reasons in detail through the analysis of chemical bonds. They were the methods to discuss the cement hydration process, especially the accurate hydration products.
3.4 SEM
SEM images of CPB at different temperatures are shown in Figure 7. The microstructure of the CPB at 10 °C (Figure 7(a)) shows obvious cement particles, indicating that most of the cement remained. In Figure 7, character symbols “a” to “d” are the observed substances ettringite (a), C-S-H gel (b), cubic portlandite (c), and Ca·Si·Al·S compound (d), respectively, according to SEM. Owing to the relatively low temperature, the amounts of needle-like ettringite, C-S-H gel, and cubic portlandite are relatively small, while the Ca·Si·Al·S polymer amount is greater. The CPB microstructure at 30 °C is more sufficient than the 10 °C hydration reaction, as shown in Figure 7(b). The needle-like ettringite also aggregates into relatively large strips while forming minute needles. Furthermore, the C-S-H gel initially grew and partly covered the surface of the tailings. The plates of cubic portlandite also exhibit a regular shape. In addition, the microscopic hydration reaction of the CPB at 50 °C is further than 30 °C, as shown in Figure 7(c). The CPB space becomes denser, and the hydration product has more typical agglomerated and porous forms.
SEM images of CPB with different pH values are shown in Figures 7(d), (e) and (f). As the pH value increases, the degree of cement reaction shows a “valley” trend, and the degree of cement hydration is greatly affected by temperature, most significantly for the three CPB groups at the same temperature and pH value of 3 and pH value of 13. However, both the quantity of CPB hydration products produced and the density are relatively similar. The CPB samples with a pH value of 3 (Figure 7(d)) exhibits more C-S-H gel and cubic portlandite but less Ca·Si·Al·S polymer and needle-like ettringite, which is consistent with the replacement of chloride ions by TG&DTG and FT-IR analysis. The CPB sample with pH value of 7 crystallizes better than the CPB hydration product with pH value of 3. However,as shown in Figure 7(e), the generation and density of the hydrated product are relatively dispersed, and the void spacing is large. In Figure 7(f), The CPB samples with a pH value of 13 samples demonstrated a great content of cubic portlandite, with a relatively high density and stronger microstructures.
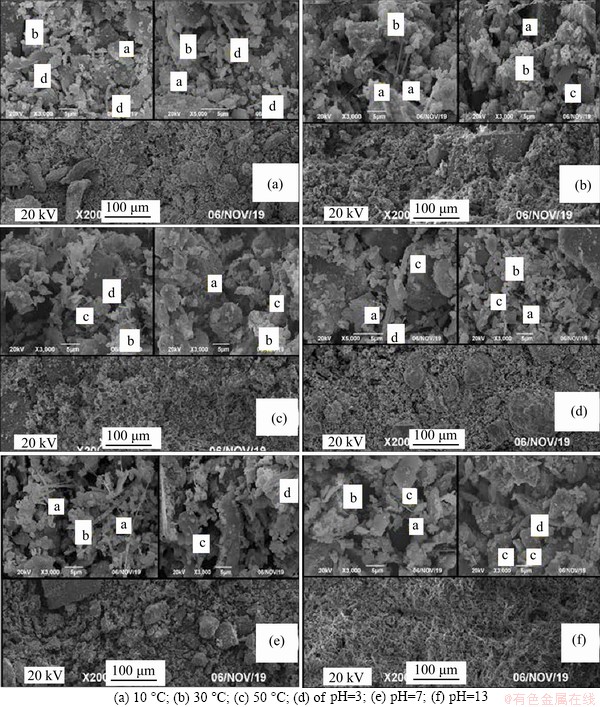
Figure 7 Microstructures of CPB at different temperatures and pH values (SEM):(“a” is needle-like ettringite; “b” is C-S-H gel; “c” is cubic portlandite; “d” is Ca·Si·Al·S compound)
In short, SEM proved the rising temperatures could increase the values of shear stress, yield stress and apparent viscosity by enhancing the cement hydration spaces and structures, so as to the pH value of 3 and 13 [75, 76]. SEM was much easier to find out and discuss the hydration products and the density of hydration spaces.
All in all, the four phenomena of rheological tests and the reasons were discussed by TG&DTG, FT-IR and SEM. TG&DTG mainly discussed the cement hydration products especially the C-S-H gel, cubic portlandite and calcium carbonate decomposition on the whole. The discussions of cement hydration products in detail were made from FT-IR. Moreover, images of SEM not only verified the main cement hydration products but also made explanations of temperatures and pH values on the rheological properties of CPB especially the hydration spaces, the rates, the structures and the density.
4 Conclusions
The effects of temperatures and pH values on the rheological properties of CPB are as follows:
Temperature affects the rheological properties of CPB to a large extent, as reflected by the shear stress, yield stress, and apparent viscosity. While the effect of pH values on the rheological properties of CPB is relatively small.
1) Increasing the curing temperature can enhance or reduce the friction between molecules and particles in CPB, which may contribute to higher shear stress, higher apparent viscosity and higher yield stress in the same pH value condition. As for the yield stress with the temperature under 20 °C and it is speculated to be due to low temperatures with slow hydration reactions which show lower yield stress. The hydration reaction is accelerated when it reaches 20 °C, the CPB mixture’s structure space begins to build. Moreover, the yield stress starts to decrease compared to that at low temperatures.The hydration reaction is gradually accelerated as temperatures increase. Particularly, the hydration products are further accumulated, the structure space is further compacted and enriched, which subsequently results in a higher temperature and yield stress. The microscopic experiments indicate that the hydration reaction of cement further develops with increasing temperature. In addition to the gradual emergence of various hydration products, the formed hydration space structure becomes increasingly dense, resulting in increased shear and yield stresses. Furthermore, the apparent viscosity is greater while the shear-thinning viscosity is lower.
2) The high shear stress of the CPB samples with pH value of 3 and 13 presumably the increase in hydration products and the intensive production of hydration space. Higher pH values lead to the increase of yield stress at temperature below 30 °C, which can be speculated that the hydration degree of cement will be further developed owing to an increase in hydroxide and sodium ions, as well as the more hydration products and denser space. At temperature over 40 °C, higher pH values lead to lower yield stress and it is speculated that anions (OH-) and cations (Na+) can act as activators to accelerate hydration reactions at a high pH value. However, more hydration products and spatial development of the CPB may limit the further rise in yield stress. Higher pH values lead to two different types of apparent viscosity curves. As the pH value increases, especially in pH value of 3 and 13, both the disintegration rate of the flocs in the CPB and molecular alignment occur more slowly, which cause high apparent viscosity in non-neutral condition. Microscopically, the degree of hydration reaction, reaction rate, and the hydration space structure are larger, faster, and more perfect in acidic and alkaline environments. In summary, the pH values are affected by temperature to some extent.
This study still lacks certain data support regarding the influence of pH values on rheology, and the related mechanism still requires subsequent research and supplementation. The mechanism of temperature affecting hydration will be further studied in our future work.
Contributors
ZHANG Qin-li and CHEN Qiu-song provided the concept. LI Yi-teng wrote the first draft of the manuscript. FENG Yan provided the analysis methods. LIU Yi-kai and WANG Dao-lin edited the draft of manuscript. All authors replied to reviewers’ comments and revised the final version.
Conflict of interest
ZHANG Qin-li, LI Yi-teng, CHEN Qiu-song, LIU Yi-kai, FENG Yan and WANG Dao-lin declare that they have no conflict of interest.
References
[1] LI Xi-bing, GONG Feng-qiang, TAO Ming, DONG Long-jun, DU Kun, CHU De -ma, ZHOU Zi-long, YIN Tu-bing. Failure mechanism and coupled static-dynamic loading theory in deep hard rock mining: A review [J]. Journal of Rock Mechanics and Geotechnical Engineering, 2017, 9(4): 767-782. DOI: 10.1016/j.jrmge.2017.04.004.
[2] LIU Yi-kai, ZHANG Qin-li, CHEN Qiu-song, QI Chong-chong, SU Zhu, HUANG Zhao-dong. Utilisation of water-washing pre-treated phosphogypsum for cemented paste backfill [J]. Minerals, 2019, 9(3): 175. DOI: 10.3390/ min9030175.
[3] POURRET O, LANGE B, BONHOURE J, COLINET G, DECREE S, MAHY G, SELECK M, SHUTCHA M, FAUCON M P. Assessment of soil metal distribution and environmental impact of mining in Katanga (Democratic Republic of Congo) [J]. Applied Geochemistry, 2016, 64: 43-55. DOI: 10.1016/j.apgeochem.2015.07.012.
[4] XUE Gai-li, YILMAZ E, SONG Wei-dong, CAO Shuai. Fiber length effect on strength properties of polypropylene fiber reinforced cemented tailings backfill specimens with different sizes [J]. Construction and Building Materials, 2020, 241: 118113. DOI: 10.1016/j.conbuildmat.2020.118113.
[5] CHEN Qiu-song, ZHANG Qin-li, FOURIE A, XIN Chen. Utilization of phosphogypsum and phosphate tailings for cemented paste backfill [J]. Journal of Environmental Management, 2017, 201: 19-27. DOI: 10.1016/j.jenvman. 2017.06.027.
[6] CHEN Qiu-song, ZHANG Qin-li, FOURIE A, CHEN Xin, QI Chong-chong. Experimental investigation on the strength characteristics of cement paste backfill in a similar stope model and its mechanism [J]. Construction and Building Materials, 2017, 154: 34-43. DOI: 10.1016/j.conbuildmat. 2017.07.142.
[7] FALL M, ADRIEN D, CELESTIN J C, POKHAREL M, TOURE M. Saturated hydraulic conductivity of cemented paste backfill [J]. Minerals Engineering, 2009, 22(15): 1307-1317. DOI: 10.1016/j.mineng.2009.08.002.
[8] CAO Shuai, XUE Gai-li, SONG Wei-dong, TENG Qing. Strain rate effect on dynamic mechanical properties and microstructure of cemented tailings composites [J]. Construction and Building Materials, 2020, 247: 118537. DOI: 10.1016/j.conbuildmat.2020.118537.
[9] YILMAZ T, ERCIKDI B, CIHANGIR F. Evaluation of the neutralization performances of the industrial waste products (IWPs) in sulphide-rich environment of cemented paste backfill [J]. Journal of Environmental Management, 2020, 258: 110037. DOI: 10.1016/j.jenvman.2019.110037.
[10] HE Yan, CHEN Qiu-song, QI Chong-chong, ZHANG Qin-li, XIAO Chong-chun. Lithium slag and fly ash-based binder for cemented fine tailings backfill [J]. Journal of Environmental Management, 2019, 248: 109282. DOI: 10.1016/j.jenvman. 2019.109282.
[11] CIHANGIR F, ERCIKDI B, KESIMAL A, OCAK S, AKYOL Y. Effect of sodium-silicate activated slag at different silicate modulus on the strength and microstructural properties of full and coarse sulphidic tailings paste backfill [J]. Construction and Building Materials, 2018, 185: 555-566. DOI: 10.1016/j.conbuildmat.2018.07.105.
[12] WU D, FALL M, CAI S J. Coupling temperature, cement hydration and rheological behaviour of fresh cemented paste backfill [J]. Minerals Engineering, 2013, 42: 76-87. DOI: 10.1016/j.mineng.2012.11.011.
[13] KOU Yun-peng, JIANG Hai-qiang, REN Lei, YILMAZ E, LI Yuan-hui. Rheological properties of cemented paste backfill with alkali-activated slag [J]. Minerals, 2020, 10(3): 288. DOI: 10.3390/min10030288.
[14] QI Chong-chong, CHEN Qiu-song, DONG Xiang-jian, ZHANG Qin-li, YASEEN Z M. Pressure drops of fresh cemented paste backfills through coupled test loop experiments and machine learning techniques [J]. Powder Technology, 2020, 361: 748-758. DOI: 10.1016/j.powtec. 2019.11.046.
[15] JIAO Hua-zhe, WANG Shu-fei, YANG Yi-xuan, CHEN Xin-ming. Water recovery improvement by shearing of gravity-thickened tailings for cemented paste backfill [J]. Journal of Cleaner Production, 2020, 245: 118882. DOI: 10.1016/ j.jclepro.2019.118882.
[16] HE Yan, ZHANG Qin-li, CHEN Qiu-song, BIAN Ji-wei, QI Chong-chong, KANG Qian, FENG Yan. Mechanical and environmental characteristics of cemented paste backfill containing lithium slag-blended binder [J]. Construction and Building Materials, 2021, 271: 121567. DOI: 10.1016/ j.conbuildmat.2020.121567.
[17] WU Di, CAI Si-jing, HUANG Gang. Coupled effect of cement hydration and temperature on rheological properties of fresh cemented tailings backfill slurry [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(9): 2954-2963. DOI: 10.1016/S1003-6326(14)63431-2.
[18] CHENG Hai-yong, WU Shun-chuan, LI Hong, ZHANG Xiao-qiang. Influence of time and temperature on rheology and flow performance of cemented paste backfill [J]. Construction and Building Materials, 2020, 231: 117117. DOI: 10.1016/j.conbuildmat.2019.117117.
[19] WANG Yong, WU Ai-xiang, RUAN Zhu-en, WANG Hong-jiang, WANG Yi-ming, JIN Fei. Temperature effects on rheological properties of fresh thickened copper tailings that contain cement [J]. Journal of Chemistry, 2018: 5082636. DOI: 10.1155/2018/5082636.
[20] FERNANDEZ-ALTABLE V, CASANOVA I. Influence of mixing sequence and superplasticiser dosage on the rheological response of cement pastes at different temperatures [J]. Cement and Concrete Research, 2006, 36(7): 1222-1230. DOI: 10.1016/j.cemconres.2006.02.016.
[21] NEHDI M, AL MARTINI S. Effect of temperature on oscillatory shear behavior of Portland cement paste incorporating chemical admixtures [J]. Journal of Materials in Civil Engineering, 2007, 19(12): 1090-1100. DOI: 10.1061/ (asce)0899-1561(2007)19: 12(1090).
[22] PETIT J Y, KHAYAT K H, WIRQUIN E. Coupled effect of time and temperature on variations of yield value of highly flowable mortar [J]. Cement and Concrete Research, 2006, 36(5): 832-841. DOI: 10.1016/j.cemconres.2005.11.001.
[23] PETIT J Y, WIRQUIN E, KHAYAT K H. Effect of temperature on the rheology of flowable mortars [J]. Cement and Concrete Composites, 2010, 32(1): 43-53. DOI: 10.1016/j.cemconcomp.2009.10.003.
[24] FRANKS G V. Zeta potentials and yield stresses of silica suspensions in concentrated monovalent electrolytes: Isoelectric point shift and additional attraction [J]. Journal of Colloid and Interface Science, 2002, 249(1): 44-51. DOI: 10.1006/jcis.2002.8250.
[25] KASHANI A, PROVIS J L, QIAO G G, VAN DEVENTER J S J. The interrelationship between surface chemistry and rheology in alkali activated slag paste [J]. Construction and Building Materials, 2014, 65: 583-591. DOI: 10.1016/ j.conbuildmat.2014.04.127.
[26] CIHANGIR F, AKYOL Y. Effect of desliming of tailings on the fresh and hardened properties of paste backfill made from alkali-activated slag [J]. Advances in Materials Science and Engineering, 2020: 4536257. DOI: 10.1155/2020/4536257.
[27] AZARIJAFARI H, KAZEMIAN A, AHMADI B, BERENJIAN J, SHEKARCHI M. Studying effects of chemical admixtures on the workability retention of zeolitic Portland cement mortar [J]. Construction and Building Materials, 2014, 72: 262-269. DOI: 10.1016/j.conbuildmat. 2014.09.020.
[28] MA Bao-guo, PENG Yi, TAN Hong-bo, JIAN Shou-wei, ZHI Zhen-zhen, GUO Yu-lin, QI Hua-hui, ZHANG Ting, HE Xing-yang. Effect of hydroxypropyl-methyl cellulose ether on rheology of cement paste plasticized by polycarboxylate superplasticizer [J]. Construction and Building Materials, 2018, 160: 341-350. DOI: 10.1016/j.conbuildmat.2017.11. 010.
[29] BELLOTTO M. Cement paste prior to setting: A rheological approach [J]. Cement and Concrete Research, 2013, 52: 161-168. DOI: 10.1016/j.cemconres.2013.07.002.
[30] BISHOP M, BARRON A R. Cement hydration inhibition with sucrose, tartaric acid, and lignosulfonate: Analytical and spectroscopic study [J]. Industrial & Engineering Chemistry Research, 2006, 45(21): 7042-7049. DOI: 10.1021/ie060806t.
[31] LEONAVICIUS D, PUNDIENE I, PRANCKEVICIENE J, KLIGYS M. Selection of superplasticisers for improving the rheological and mechanical properties of cement paste with CNTs [J]. Construction and Building Materials, 2020, 253: 119182. DOI: 10.1016/j.conbuildmat.2020.119182.
[32] TIAN Hong-wei, KONG Xiang-ming, SU Tong, WANG Dong-min. Comparative study of two PCE superplasticizers with varied charge density in Portland cement and sulfoaluminate cement systems [J]. Cement and Concrete Research, 2019, 115: 43-58. DOI: 10.1016/j.cemconres. 2018.10.003.
[33] CHEN Xin, SHI Xiu-zhi, ZHOU Jian, YU Zhi, HUANG Pei-sheng. Determination of mechanical, flowability, and microstructural properties of cemented tailings backfill containing rice straw [J]. Construction and Building Materials, 2020, 246: 118520. DOI: 10.1016/j.conbuildmat.2020.118520.
[34] CHEN Qiu-song, ZHANG Qin-li, QI Chong-chong, FOURIE A, XIAO Chong-chun. Recycling phosphogypsum and construction demolition waste for cemented paste backfill and its environmental impact [J]. Journal of Cleaner Production, 2018, 186: 418-429. DOI: 10.1016/j.jclepro.2018.03.131.
[35] PANCHAL S, DEB D, SREENIVAS T. Variability in rheology of cemented paste backfill with hydration age, binder and superplasticizer dosages [J]. Advanced Powder Technology, 2018, 29(9): 2211-2220. DOI: 10.1016/j.apt.2018.06.005.
[36] Changsha Water Group Co., Ltd. Monthly report of routine water quality index of water plant [EB/OL]. [2019-08-26]. http//www.supplywater.com/tstz-79-43.aspx. (in Chinese)
[37] HAGER I. Behaviour of cement concrete at high temperature [J]. Bulletin of the Polish Academy of Sciences: Technical Sciences, 2013, 61(1): 145-154. DOI: 10.2478/bpasts-2013-0013.
[38] MIRZA J, SALEH K, LANGEVIN M A, MIRZA S, BHUTTA M A R, TAHIR M M. Properties of microfine cement grouts at 4 °C, 10 °C and 20 °C [J]. Construction and Building Materials, 2013, 47: 1145-1153. DOI: 10.1016/j.conbuildmat. 2013.05.026.
[39] FEI Jiang-chi, MIN Xiao-bo, WANG Zhen-xing, PANG Zhi-hua, LIANG Yan-jie, KE Yong. Health and ecological risk assessment of heavy metals pollution in an antimony mining region: A case study from South China [J]. Environmental Science and Pollution Research, 2017, 24(35): 27573-27586. DOI: 10.1007/s11356-017-0310-x.
[40] WU Di, DENG Teng-fei, ZHAO Run-kang. A coupled THMC modeling application of cemented coal gangue-fly ash backfill [J]. Construction and Building Materials, 2018, 158: 326-336. DOI: 10.1016/j.conbuildmat.2017.10.009.
[41] WU Di, SUN Guang-hua, LIU Yu-cheng. Modeling the thermo-hydro-chemical behavior of cemented coal gangue-fly ash backfill [J]. Construction and Building Materials, 2016, 111: 522-528. DOI: 10.1016/j.conbuildmat.2016.02.179.
[42] IPAVEC A, VUK T, GABROVSEK R, KAUCIC V. Chloride binding into hydrated blended cements: The influence of limestone and alkalinity [J]. Cement and Concrete Research, 2013, 48: 74-85. DOI: 10.1016/j.cemconres.2013.02.010.
[43] WANG Ke-jin, SHAH S P, MISHULOVICH A. Effects of curing temperature and NaOH addition on hydration and strength development of clinker-free CKD-fly ash binders [J]. Cement and Concrete Research, 2004, 34(2): 299-309. DOI: 10.1016/j.cemconres.2003.08.003.
[44] WANG Yong, WU Ai-xiang, RUAN Zhu-en, WANG Zhi-hui, WEI Zong-su, YANG Gang-feng, WANG Yi-ming. Reconstructed rheometer for direct monitoring of dewatering performance and torque in tailings thickening process [J]. International Journal of Minerals, Metallurgy and Materials, 2020, 27(11): 1430-1437. DOI: 10.1007/s12613-020-2116-y.
[45] ASSAAD J J, HARB J, MAALOUF Y. Effect of vane configuration on yield stress measurements of cement pastes [J]. Journal of Non-Newtonian Fluid Mechanics, 2016, 230: 31-42. DOI: 10.1016/j.jnnfm.2016.01.002.
[46] SAAK A W, JENNINGS H M, SHAH S P. A generalized approach for the determination of yield stress by slump and slump flow [J]. Cement and Concrete Research, 2004, 34(3): 363-371. DOI: 10.1016/j.cemconres.2003.08.005.
[47] BARNES H A, NGUYEN Q D. Rotating vane rheometry—A review [J]. Journal of Non-Newtonian Fluid Mechanics, 2001, 98(1): 1-14. DOI: 10.1016/S0377-0257(01)00095-7.
[48] YUAN Qiang, ZHOU Da-jun, KHAYAT K H, FEYS D, SHI Cai-jun. On the measurement of evolution of structural build-up of cement paste with time by static yield stress test vs. small amplitude oscillatory shear test [J]. Cement and Concrete Research, 2017, 99: 183-189. DOI: 10.1016/j.cemconres. 2017.05.014.
[49] FEYS D, VERHOEVEN R, DE SCHUTTER G. Fresh self compacting concrete, a shear thickening material [J]. Cement and Concrete Research, 2008, 38(7): 920-929. DOI: 10.1016/j.cemconres.2008.02.008.
[50] LUZ A P, PANDOLFELLI V C. Halting the calcium aluminate cement hydration process [J]. Ceramics International, 2011, 37(8): 3789-3793. DOI: 10.1016/j.ceramint.2011.06.034.
[51] DANNER T, JUSTNES H, GEIKER M, LAUTEN R A. Phase changes during the early hydration of Portland cement with Ca-lignosulfonates [J]. Cement and Concrete Research, 2015, 69: 50-60. DOI: 10.1016/j.cemconres.2014.12.004.
[52] FENG Yan, CHEN Qiu-song, ZHOU Yan-long, YANG Qi-xing, ZHANG Qin-li, JIANG Liang, GUO Hong-quan. Modification of glass structure via CaO addition in granulated copper slag to enhance its pozzolanic activity [J]. Construction and Building Materials, 2020, 240: 117970. DOI: 10.1016/j.conbuildmat.2019.117970.
[53] CHEN Yan-yan, FURMANN A, MASTALERZ M, SCHIMMELMANN A. Quantitative analysis of shales by KBr-FTIR and micro-FTIR [J]. Fuel, 2014, 116: 538-549. DOI: 10.1016/j.fuel.2013.08.052.
[54] CHEN Xin, SHI Xiu-zhi, ZHANG Shu, CHEN Hui, ZHOU Jian, YU Zhi, HUANG Pei-sheng. Fiber-reinforced cemented paste backfill: The effect of fiber on strength properties and estimation of strength using nonlinear models [J]. Materials, 2020, 13(3): 718. DOI: 10.3390/ma13030718.
[55] JIANG Guan-zhao, WU Ai-xiang, WANG Yi-ming, LI Jian-qiu. The rheological behavior of paste prepared from hemihydrate phosphogypsum and tailing [J]. Construction and Building Materials, 2019, 229: 116870. DOI: 10.1016/ j.conbuildmat.2019.116870.
[56] CHEN Qiu-song, SUN Shi-yuan, LIU Yi-kai, QI Chong-chong, ZHOU Hui-bo, ZHANG Qin-li. Experimental and numerical study on immobilization and leaching characteristics of fluoride from phosphogypsum based cemented paste backfill [J]. International Journal of Minerals, Metallurgy and Materials, 2021. DOI: 10.1007/s12613-021-2274-6.
[57] ROSHANI A, FALL M. Rheological properties of cemented paste backfill with nano-silica: Link to curing temperature [J]. Cement and Concrete Composites, 2020, 114: 103785. DOI: 10.1016/j.cemconcomp.2020.103785.
[58] BETIOLI A M, HOPPE FILHO J, CINCOTTO M A, GLEIZE P J P, PILEGGI R G. Chemical interaction between EVA and Portland cement hydration at early-age [J]. Construction and Building Materials, 2009, 23(11): 3332-3336. DOI: 10.1016/j.conbuildmat.2009.06.033.
[59] ZATTA L, GARDOLINSKI J E F D C, WYPYCH F. Raw halloysite as reusable heterogeneous catalyst for esterification of lauric acid [J]. Applied Clay Science, 2011, 51(1, 2): 165-169. DOI: 10.1016/j.clay.2010.10.020.
[60] ZAJAC M, SKOCEK J, DURDZINSKI P, BULLERJAHN F, SKIBSTED J, BEN HAHA M. Effect of carbonated cement paste on composite cement hydration and performance [J]. Cement and Concrete Research, 2020, 134: 106090. DOI: 10.1016/j.cemconres.2020.106090.
[61] KUMAR A, SANT G, PATAPY C, GIANOCCA C, SCRIVENER K L. The influence of sodium and potassium hydroxide on alite hydration: Experiments and simulations [J]. Cement and Concrete Research, 2012, 42(11): 1513-1523. DOI: 10.1016/j.cemconres.2012.07.003.
[62] MOTA B, MATSCHEI T, SCRIVENER K. Impact of NaOH and Na2SO4 on the kinetics and microstructural development of white cement hydration [J]. Cement and Concrete Research, 2018, 108: 172-185. DOI: 10.1016/j.cemconres.2018.03.017.
[63] ALONSO S, PALOMO A. Calorimetric study of alkaline activation of calcium hydroxide-metakaolin solid mixtures [J]. Cement and Concrete Research, 2001, 31(1): 25-30. DOI: 10.1016/S0008-8846(00)00435-X.
[64] BAKHAREV T, SANJAYAN J G, CHENG Y B. Resistance of alkali-activated slag concrete to carbonation [J]. Cement and Concrete Research, 2001, 31(9): 1277-1283. DOI: 10.1016/S0008-8846(01)00574-9.
[65] EL-ALFI E A, GADO R A. Preparation of calcium sulfoaluminate-belite cement from marble sludge waste [J]. Construction and Building Materials, 2016, 113: 764-772. DOI: 10.1016/j.conbuildmat.2016.03.103.
[66] GARCIA LODEIRO I, MACPHEE D E, PALOMO A, FERNANDEZ-JIMENEZ A. Effect of alkalis on fresh C-S-H gels. FTIR analysis [J]. Cement and Concrete Research, 2009, 39(3): 147-153. DOI: 10.1016/j.cemconres.2009.01.003.
[67] ELKHADIRI I, PALACIOS M, PUERTAS F. Effect of curing temperatura on hydration process of different cement [J]. Ceramics-Silikaty. 2009, 53: 65-75.
[68] VARAS M J, ALVAREZ DE BUERGO M, FORT R. Natural cement as the precursor of Portland cement: Methodology for its identification [J]. Cement and Concrete Research, 2005, 35(11): 2055-2065. DOI: 10.1016/j.cemconres.2004.10.045.
[69] YLMEN R, JAGLID U. Carbonation of Portland cement studied by diffuse reflection Fourier transform infrared spectroscopy [J]. International Journal of Concrete Structures and Materials, 2013, 7(2): 119-125. DOI: 10.1007/s40069-013-0039-y.
[70] KAPELUSZNA E, KOTWICA L, ROZYCKA A, GOLEK L. Incorporation of Al in C-A-S-H gels with various Ca/Si and Al/Si ratio: Microstructural and structural characteristics with DTA/TG, XRD, FTIR and TEM analysis [J]. Construction and Building Materials, 2017, 155: 643-653. DOI: 10.1016/ j.conbuildmat.2017.08.091.
[71] DONATELLO S, PALOMO A, FERNANDEZ-JIMENEZ A. Durability of very high volume fly ash cement pastes and mortars in aggressive solutions [J]. Cement and Concrete Composites, 2013, 38: 12-20. DOI: 10.1016/j.cemconcomp. 2013.03.001.
[72] PAJARES I, MART1NEZ-RAM1REZ S, BLANCO-VARELA M T. Evolution of ettringite in presence of carbonate, and silicate ions [J]. Cement and Concrete Composites, 2003, 25(8): 861-865. DOI: 10.1016/S0958-9465(03)00113-6.
[73] WANG Dao-lin, ZHANG Qin-li, CHEN Qiu-song, QI Chong-chong, FENG Yan, XIAO Chong-chun. Temperature variation characteristics in flocculation settlement of tailings and its mechanism [J]. International Journal of Minerals, Metallurgy and Materials, 2020, 27(11): 1438-1448. DOI: 10.1007/ s12613-020-2022-3.
[74] CHEN Sheng-li, SUN Shen-mei, CHEN Xiao-long, ZHONG Kai-hong, SHAO Qiang, XU Hai-jun, WEI Jiang-xiong. Effects of core-shell polycarboxylate superplasticizer on the fluidity and hydration behavior of cement paste [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 590: 124464. DOI: 10.1016/j.colsurfa.2020.124464.
[75] JAKOB C, JANSEN D, UKRAINCZYK N, KOENDERS E, POTT U, STEPHAN D, NEUBAUER J. Relating ettringite formation and rheological changes during the initial cement hydration: A comparative study applying XRD analysis, rheological measurements and modeling [J]. Materials (Basel, Switzerland), 2019, 12(18): E2957. DOI: 10.3390/ ma12182957.
[76] JIAO Deng-wu, SHI Cai-jun, YUAN Qiang, AN Xiao-peng, LIU Yu, LI Huang. Effect of constituents on rheological properties of fresh concrete-A review [J]. Cement and Concrete Composites, 2017, 83: 146-159. DOI: 10.1016/ j.cemconcomp.2017.07.016.
(Edited by ZHENG Yu-tong)
中文导读
温度和pH值对全尾砂膏体流变特性的影响
摘要:本文研究了在不同温度和pH值条件下,温度和pH值对全尾砂膏体流变特性的影响及其影响机制。在不同温度和pH值的条件下制备全尾砂膏体,温度控制分别设置在10,20,30,40和50 °C,pH值控制分别设置为3,7和13。对制备完成的全尾砂膏体进行流变剪切试验、热重实验(TG&DTG),傅立叶变换红外光谱实验(FT-IR)和扫描电子显微镜分析(SEM)。研究结果表明,温度对全尾砂膏体的流变特性有显著影响,而pH值对全尾砂膏体的流变特性的影响相对较小。在相同的pH值条件下,较高温度(超过20 °C)的全尾砂膏体易产生较高的剪切应力、屈服应力和表观黏度,但过高的温度(50 °C)无法达到更高的表观黏度。在同一温度、pH值为3和13时的非中性环境下,全尾砂膏体的剪切应力和表观黏度较中性条件都有所增强。相同温度下(低于50 °C),pH值的升高会导致全尾砂膏体产生两种不同的屈服应力和表观黏度曲线。微观分析表明,全尾砂膏体的流变特性受温度和pH值的影响,温度和pH值会影响全尾砂膏体中水泥的水化过程、水化速率及水化产物的空间结构。
关键词:全尾砂膏体;流变特性;温度;pH值;水泥水化;微观分析
Foundation item: Project(2019zzts678) supported by the Fundamental Research Funds for the Central Universities, China
Received date: 2020-10-30; Accepted date: 2021-03-10
Corresponding author: CHEN Qiu-song, PhD, Associate Professor; E-mail: qiusong.chen@csu.edu.cn; ORCID: https://orcid.org/0000-0002-7588-8097

