含锑难处理金矿工艺矿物学特征及臭氧氧化预处理浸锑
来源期刊:中国有色金属学报(英文版)2017年第8期
论文作者:郭学益 辛云涛 王浩 田庆华
文章页码:1888 - 1895
关键词:含锑难处理金矿;臭氧;预处理;矿物学特征;提锑
Key words:antimony-bearing refractory gold concentrates; ozone; pretreatment; mineralogical characterization; antimony extraction
摘 要:针对复杂难处理金矿的矿物学特征和盐酸体系臭氧氧化预处理提锑开展研究。工艺矿物学研究表明,矿物中主要存在辉锑矿、毒砂、黄铁矿和脉石,67.42%的金被包裹在硫化物之中。开展盐酸体系中臭氧氧化提锑的研究,考察反应温度、液固比、盐酸浓度和搅拌速度等因素对提锑过程的影响,在优化实验条件下,锑的浸出率可达93.75%。经过臭氧氧化预处理过程,矿物中的锑得到有效回收,同时包裹在辉锑矿和部分黄铁矿中的金得到释放和暴露。
Abstract: The mineralogical characterization of antimony-bearing refractory gold concentrates and the antimony extraction by ozone in HCl solution were investigated. The mineralogical study shows that there exist stibnite(Sb2S3), arsenopyrite(FeAsS), pyrite(FeS2) and quartz in the concentrates, and the gold is mainly (67.42%) encapsulated in sulfides. The antimony extraction by ozone in hydrochloric acid was employed and the influences of temperature, liquid/solid ratio, HCl concentration and stirring speed on the extraction of antimony were investigated. High antimony extraction (93.75%) is achieved under the optimized conditions. After the pretreatment by ozone, the antimony is recovered efficiently and the gold is enriched in the leaching residue.
Trans. Nonferrous Met. Soc. China 27(2017) 1888-1895
Xue-yi GUO1,2, Yun-tao XIN1,2, Hao WANG1,2, Qing-hua TIAN1,2
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Cleaner Metallurgical Engineering Research Center, China Nonferrous Metals Industry Association, Changsha 410083, China
Received 24 August 2016; accepted 14 June 2017
Abstract: The mineralogical characterization of antimony-bearing refractory gold concentrates and the antimony extraction by ozone in HCl solution were investigated. The mineralogical study shows that there exist stibnite(Sb2S3), arsenopyrite(FeAsS), pyrite(FeS2) and quartz in the concentrates, and the gold is mainly (67.42%) encapsulated in sulfides. The antimony extraction by ozone in hydrochloric acid was employed and the influences of temperature, liquid/solid ratio, HCl concentration and stirring speed on the extraction of antimony were investigated. High antimony extraction (93.75%) is achieved under the optimized conditions. After the pretreatment by ozone, the antimony is recovered efficiently and the gold is enriched in the leaching residue.
Key words: antimony-bearing refractory gold concentrates; ozone; pretreatment; mineralogical characterization; antimony extraction
1 Introduction
Gold is highly contaminated and encapsulated in sulfide matrix in refractory gold ores, and the extraction efficiency of gold by direct cyanidation leaching is low because of the structural characteristics [1-3], at the same time the simple mechanical process cannot liberate the gold [4]. Pretreatment is usually adopted for refractory gold ores before gold leaching. Since the sulfides in ores are decomposed by pretreatment process, the encapsulated gold could be liberated and then extracted. Pretreatments of refractory gold ores include pyrometallurgical treatments and hydrometallurgical ones. The pyrometallurgical pretreatments mainly refer to roasting, and the hydrometallurgical ones could be divided into pressure oxidation, bio-oxidation and chemical treatments according to different oxidation methods [5-8]. Owing to the clean, residue-free and efficient characteristics, ozone has been used as the oxidant in the hydrometallurgical leaching process [9-12].  et al [13] treated the flotation tailings of two kinds of pyrite with ozone as oxidant before cyanide leaching. QIAN et al [14] added ozone into refractory gold with ferric chloride as cooperative oxidant to break the cover of sulfides on gold.
et al [13] treated the flotation tailings of two kinds of pyrite with ozone as oxidant before cyanide leaching. QIAN et al [14] added ozone into refractory gold with ferric chloride as cooperative oxidant to break the cover of sulfides on gold.
There are many researches on pretreatment of pyrite-type refractory gold ores by ozone, while little information on pretreatment of antimony-bearing refractory gold ores or research on stibnite leaching using ozone as oxidant has been reported. Mineralogical study could reveal the characterization of ores and show the interrelationship of minerals in the ores, which is usually used to analyze the refractory gold ores for pretreatment and efficient gold extraction and provide the basis information for follow-up process [15,16]. In this work, the mineralogical characterization of antimony-bearing refractory gold concentrates was studied and pretreatment by ozone in hydrochloric acid to extract antimony was investigated.
2 Experimental
2.1 Materials and characterization
The concentrates were crushed to a size of 100% (<0.074 mm), and then the concentrations of metal elements were determined by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES, PS-6, Baird, USA) and X-ray fluorescence (XRF, S0902724, Rigaku, Japan). The mineralogical composition of the concentrates was identified by X-ray diffraction (XRD, S0902240, Rigaku, Japan). At the same time, the concentrates were analyzed by scanning electron microscope (SEM, JSM-6360LV, JEOL, Japan), energy dispersive spectrometer (EDS, EDX-GENESIS, Ametek, USA), electron microprobe analysis (EMPA, 1720H, Shimadzu, Japan) and some chemistry methods (listed in part 3.1, which meets the national standard of People’s Republic of China).
Analytical grade hydrochloric acid (36%-38% (w/w)) was used to prepare the HCl solution. Industrial grade oxygen was used to produce ozone for the leaching process by an ozonizer (OZOMJB-80B, ANQIU OZOMAX EQUIPMENT, China) and the mass flow of the ozone was 120 mg/L with 2.0 L/min feed gas.
The chemical composition of the concentrates is listed in Table 1. The XRD analysis in Fig. 1 shows that stibnite (Sb2S3), arsenopyrite (FeAsS), pyrite (FeS2) and quartz are the major mineral phases.
Table 1 Composition of antimony-bearing gold concentrates (<0.074 mm) (mass fraction, %)

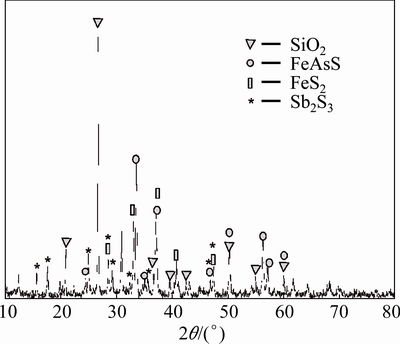
Fig. 1 XRD pattern of antimony-bearing refractory gold concentrates
2.2 Experimental procedure
The experiments were carried out in a slender beaker (800 mL) heated by a thermostat water bath (DF-101B, YUHUA, China) equipped with a mechanical stirrer and a digital controller unit. Ozonizer provided a constant amount of ozone during the experiments and the gas (a mixture of ozone and oxygen) flow rate was controlled by a gas flowmeter.
As leaching agent, 400 mL of hydrochloric acid with the required mole ratio diluted with water was added to the beaker. When the temperature reached the set value, a certain amount of the antimony-bearing refractory gold concentrates were added, and at the same time the ozone gas was pumped into the solution through a glass pipe. A condenser was used to prevent evaporation during the experiments, and 5.0 mL of the liquor sample was collected to assay by ICP-AES at the specific time, and then 5.0 mL of the HCl solution was added to the system. After the leaching treatment, the slurry was withdrawn from the reactor and processed by suction filtration. The volume of the filtered pregnant solution was measured by a measuring cylinder. The filter cake was washed twice with 400 mL distilled water, and then dried at 80 °C for 24 h.
3 Results and discussion
3.1 Mineralogical characterization of antimony- bearing refractory gold concentrates
The tandem chemistry methods were adopted in a certain order to determine the content of gold in different phases [17]. The exposed gold was directly extracted in solution of I2-KI (5%-10%) at room temperature for 4.0 h. After that, the concentrates were put in 20% acetic acid (HAC) solution at room temperature for 2.0 h to extract the gold in carbonate. Then, 3.0 mol/L HCl solution with some drops of 30% SnCl2 solution was used for extraction of gold in oxides at 90 °C for 8.0 h. After the treatments above, the gold encapsulated in sulfides was extracted by solution of EDTA+H2O2 at room temperature for 3.0 d. The gold, left in residue after these leaching processes above, was enclosed in gangue. These leaching processes were carried out in thermostat water bath with stirring speed of 300 r/min, and the liquid-to-solid ratio was kept at 10:1. The results are listed in Table 2.
Table 2 Contents of gold in different phases of antimony- bearing refractory gold concentrates (mass fraction, %)
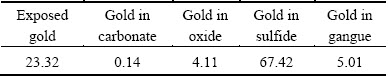
Table 2 shows that about 67.5% gold is encapsulated in sulfides in concentrates and 23.32% gold is exposed, while a very little of gold is distributed in carbonates and oxides. So, it is difficult to extract gold directly by traditional processes without pretreatments.
The concentrates were detected by EMPA to understand the distribution of main elements in the concentrates, as shown in Fig. 2. According to the result of EMPA, the distribution of dark particles agrees with the distribution of silicon and distribution of hoar particles is in agreement with the distribution of elements antimony, iron and arsenic, respectively, so the dark particles are gangue and the hoar particles stand for sulfides. The distribution of gold could be seen in Fig. 2(f), which is in agreement with the distribution of antimony, iron and arsenic, meaning that the gold is closely combined with the sulfides.
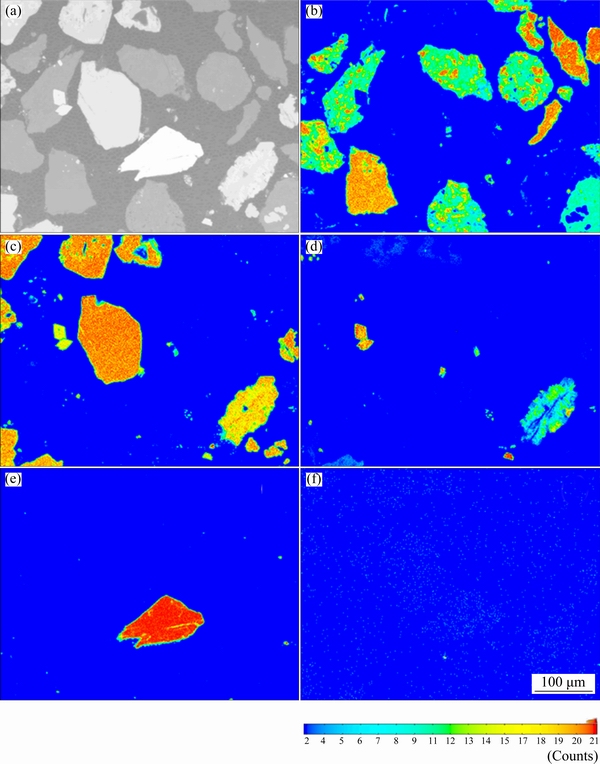
Fig. 2 EMPA pattern (a) and corresponding distribution of elements Si (b), Fe (c), As (d), Sb (e) and Au (f) of antimony-bearing refractory gold concentrates
Based on the results above, the raw materials are typical refractory gold concentrates with complex sulfides, in which the gold is encapsulated and could not be extracted directly without pretreatments.
3.2 Antimony extraction by ozone
According to the mineralogical characterization of antimony-bearing refractory gold concentrates, it is obvious that the gold is mainly enclosed in stibnite, arsenopyrite and pyrite. Before the extraction of gold, pretreatments on releasing gold in sulfides need to be operated. Besides the gold, the valuable antimony in the concentrates also needs to be recovered. The pyrometallurgical process usually results in serious environmental pollution and high energy consumption [18]. While the hydrometallurgical process is considered as an environmentally friendly technique which has attracted much attention due to its potentials in dealing with low grade complex sulfides [19-21]. In this study, the oxidation by ozone is adopted to decompose sulfides in the antimony-bearing refractory gold concentrates.
In the acidic chloride system, hydrochloric acid is used as the lixivant in conjunction with oxidant for antimony extraction from stibnite. In the process proposed by YANG and WU [22], SbCl5 was used as the oxidant to obtain the SbCl3 solution at 85 °C. The antimony extraction from the pregnant leach solution in acidic chloride system was summarized by DU and TANG [23]. Our team has worked on the stibnite oxidation-leaching by ozone [24], and the refractory gold concentrates in this study also involve oxidation-leaching of stibnite, so we adopt the same method in the pretreatment.
The proposed reactions of leaching referring to stibnite (Sb2S3), pyrite (FeS2) and arsenopyrite (FeAsS) in hydrochloric acid dissolution by the authors are shown in Eqs. (1)-(6) considering the formation of elemental sulfur and sulfate:
Sb2S3+2iCl-+3H++12O3=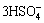 +
+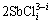 +12O2 (1)
+12O2 (1)
FeS2+H2O+7O3=Fe2++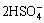 +7O2 (2)
+7O2 (2)
2FeAsS+3H2O+4H++5O3=2Fe2++2H3AsO3+2H2SO4+2O2 (3)
Sb2S3+2iCl-+6H++3O3=3S+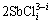 +3H2O+3O2 (4)
+3H2O+3O2 (4)
FeS2+2H++O3=Fe2++2S+O2+H2O (5)
2FeAsS+H2O+4H++3O3=2Fe2++2H3AsO3+2S+2O2 (6)
The effects of temperature, liquid/solid (L/S) ratio, acid concentration and stirring speed on the leaching process were investigated and the comparison of ozone gas and oxygen was also conducted in this study.
3.2.1 Effects of temperature
The effect of temperature on the leaching efficiencies of antimony, iron, sulfur and arsenic is shown in Fig. 3, and in the experiments the concentration of HCl was 4.0 mol/L, L/S ratio was 10 mL/g and stirring speed was kept at 300 r/min. The results indicate that the leaching efficiency of antimony increases rapidly from 22.0% at 25 °C to 93.6% at 85 °C. The leaching efficiency of iron increases from 9.9% at 25 °C to 24.6% at 85 °C, the leaching efficiency of sulfur slightly increases from 7.6% at 25 °C to 25.0% at 85 °C, and the leaching efficiency of arsenic slightly increases from 6.0% at 25 °C to 10.8% at 85 °C. This is because the mass transfer between concentrates, ozone gas and lixivium is promoted with the increase of temperature, so the leaching efficiency increases with the increase of temperature. The temperature of 85 °C is chosen and used in remaining experiments.
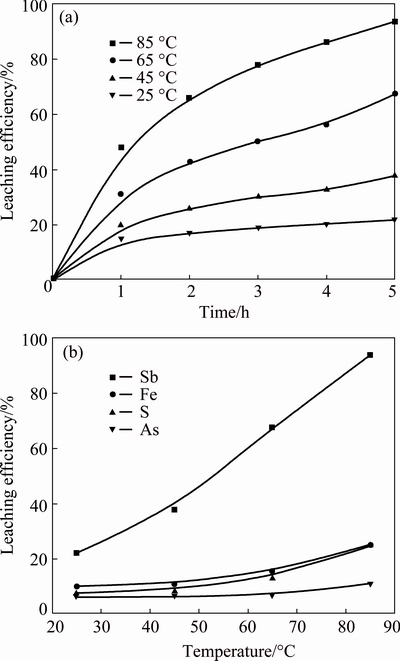
Fig. 3 Leaching efficiency of antimony vs time at different temperatures (a) and effects of temperature on leaching efficiencies of antimony, iron, sulfur and arsenic (b)
It should be noted that the gold could also be extracted in the presence of oxidants (ozone and oxygen) and complexant (Cl-), so in this leaching process it is reasonable to pay attention to the behavior of gold. In the experiments, the potential of system in the process was detected by electrochemical workstation (CHI600, CH Instruments Ins, USA) with Pt electrode and saturated calomel electrode, and the potential-pH diagram of gold was drawn according to related data [25], as shown in Fig. 4. From Fig. 4, it is easily known that when the ozone gas was pumped into solution, the potential increased sharply from 0.49 to 0.71 V, and then grew slightly in the leaching process. The fluctuation of potential after 3.0 h was attributed to the transformation of Fe2+/Fe3+ by oxidation with ozone or oxygen, as shown in Eq. (7), so the fluctuation becomes stronger as the concentration of Fe increases. It is easily known from the potential-pH diagram in Fig. 4, the gold will be extracted when the system potential surpasses 0.86 V, but the potential in the leaching process was kept under 0.85 V, so the gold was considered to stay in the residue.
Fe3++e=Fe2+ (φ=0.771 V) (7)
In order to figure out the effect of ozone on the leaching process, the comparison between pretreatment by pure oxygen gas and pretreatment by ozone gas was conducted, and the operation conditions in these two pretreatments were kept consistent. The results of comparison are shown in Fig. 5. In Fig. 5 the result of ozone gas is in dotted line and that of oxygen gas is in solid line. The leaching efficiencies of antimony, iron, sulfur and arsenic by oxygen and by ozone gas are on the same trajectory, but the leaching efficiencies by oxygen gas are obviously lower than that by ozone gas, meaning that the ozone in gas plays an important role in the leaching process and promotes the oxidation reactions. The pretreatment by ozone gas is more effective and quick than that by pure oxygen gas.
3.2.2 Effect of liquid/solid ratio
Figure 6 presents the effect of L/S ratio on the leaching efficiencies of antimony, iron, sulfur and arsenic. The volume of leaching agent was kept at 400 mL and L/S ratio was changed by adjusting the mass of concentrate in the experiments, at the same time the concentration of HCl was 4.0 mol/L and the stirring speed was kept at 300 r/min. The leaching efficiencies of antimony and sulfur increase with the increase of L/S ratio from 6:1 to 10:1 and then keep constant by further increase of L/S ratio. The leaching efficiencies of iron and arsenic rise slightly with the increase of L/S ratio. The increase of L/S ratio would enhance the mass transfer process and the particles in concentrates could be surrounded by more reactants when the L/S ratio is higher, so the leaching efficiency increases with the increase of L/S ratio. Considering the economy and leaching efficiency of antimony, the L/S ratio of 10:1 is chosen as the optimal condition.
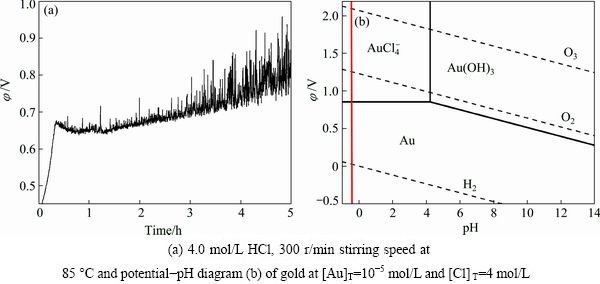
Fig. 4 Potential of system over time in leaching process with operating conditions

Fig. 5 Leaching efficiency of antimony vs time at different temperatures (a) and effects of temperature on leaching efficiencies of antimony, iron, sulfur and arsenic (b)
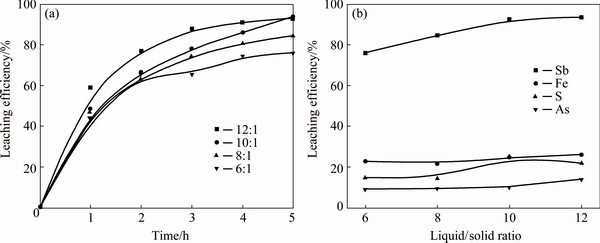
Fig. 6 Leaching efficiency of antimony vs time at different L/S ratios (a) and effects of L/S ratio on leaching efficiencies of antimony, iron, sulfur and arsenic (b)
3.2.3 Effect of acid concentration
It is well known that the antimony is in the form of SbClx complex in the hydrochloric acid solution and the Cl- could enhance the stability and solubility of antimony because of the complexation [18]. Figure 7 shows the results of leaching efficiencies of antimony, iron, sulfur and arsenic in HCl solution with different concentrations in the range of 1.0-4.0 mol/L, in the experiments the leaching temperature was 85 °C, the L/S ratio was 10 mL/g and the stirring speed was kept at 300 r/min. According to the results, the leaching efficiency of antimony increases significantly from 15.4% to 93.6% with the increase of HCl concentration from 1.0 to 3.0 mol/L, and then increases slightly when the concentration of HCl continues to reach 4.0 mol/L, whereas the leaching efficiencies of iron, sulfur and arsenic increase slowly in this range of concentrations. In this study the concentration of 3.0 mol/L is chosen to be optimal for the remaining experiments.
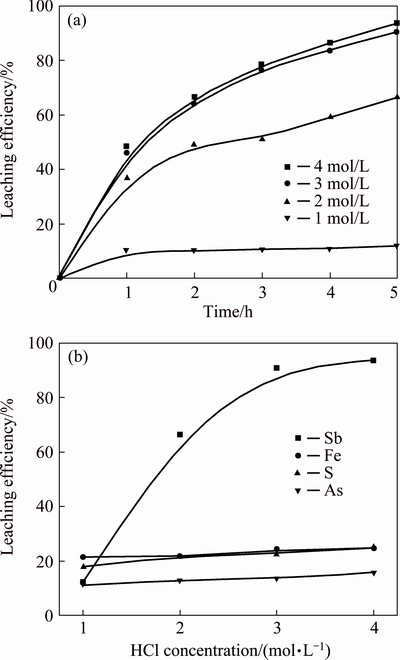
Fig. 7 Leaching efficiency of antimony vs time at different HCl concentrations (a) and effects of HCl concentration on leaching efficiencies of antimony, iron, sulfur and arsenic (b)
3.2.4 Effect of stirring speed
Figure 8 shows the results of leaching efficiencies of antimony, iron, sulfur and arsenic with the stirring speed of 300-900 r/min, in the experiments, the temperature was 85 °C, the concentration of HCl was 3.0 mol/L and the L/S ratio was 10 mL/g. The leaching efficiency of antimony increases slightly from 90.50% to 93.75% with the increase of stirring speed from 300 to 500 r/min, and then keeps constant when the stirring speed subsequently increases to 900 r/min. The leaching efficiency of iron increases from 24.64% to 37.50% when the stirring speed increases from 300 to 700 r/min, then keeps constant by further increase of stirring speed, and the leaching efficiencies of sulfur and arsenic rise slightly with the increase of stirring speed. The leaching process involves gas-liquid-solid three-phase reaction and stirring mainly affects mass transfer. Increase of stirring speed could enhance the interface reactions of reactants. The leaching efficiency of antimony quickly reached 90.64% at 3.0 h with stirring speed of 900 r/min, so 900 r/min is chosen as the optimal condition.
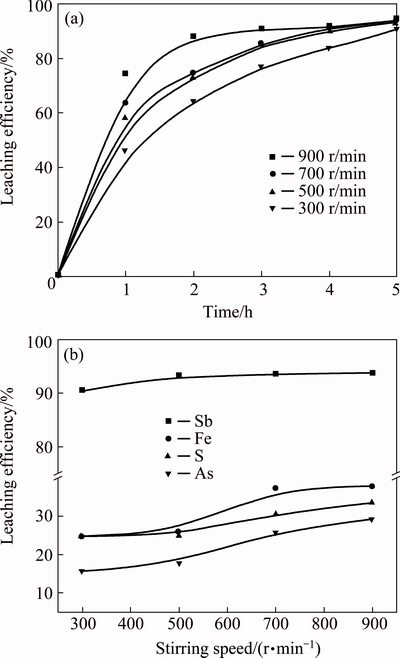
Fig. 8 Leaching efficiency of antimony vs time at different stirring speeds (a) and effects of stirring speed on leaching efficiencies of antimony, iron, sulfur and arsenic (b)
3.3 Characterization of antimony-bearing refractory gold concentrates after antimony extraction
35.7 g residue (residue rate of 89.25%) was obtained in the experiment with operating conditions: 85 °C, 3.0 mol/L HCl, 900 r/min, gas flow rate of 2.0 L/min, L/S ratio of 10 mL/g and 5.0 h. The composition of the residue is listed in Table 3. Little antimony is left and the gold is mildly enriched from 55 to 61 g/t. The sulfides in the refractory gold concentrates reduce while the gangue increases after the pretreatment.
Table 3 Composition of residue (mass fraction, %)

No stibnite is detected in the residue by XRD (see Fig. 9), which means that after the pretreatment the antimony is substantially recovered. At the same time no sulfur is found in the residue, meaning that the sulfur in sulfides was transformed into sulfate instead of elemental sulfur, hence there is no secondary encapsulation in the pretreatment process.
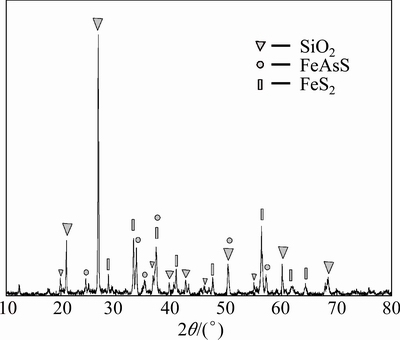
Fig. 9 XRD pattern of leaching residue
4 Conclusions
1) Mineralogical characterization of the concentrates shows that the concentrates are typical refractory gold concentrates with a plenty of sulfides which enclose the gold. There mainly exist stibnite (Sb2S3), arsenopyrite (FeAsS), pyrite (FeS2) and quartz in concentrates. 67.42% gold is distributed in sulfides and 23.32% gold is exposed.
2) The leaching efficiency of antimony increases with increasing temperature, hydrochloric acid concentration, stirring speed and L/S ratio. Under optimal conditions, 93.75% antimony could be extracted.
3) There is no elemental sulfur generated in the residue, which means no encapsulation and easy extraction of gold after the pretreatment. The sulfides decrease while gangue increases in leaching residue after the pretreatment, and the gold in stibnite is released. The residue rate is 89.25% and the concentration of gold is enriched 61 g/t after antimony extraction.
References
[1] SABA M, MOHAMMADYOUSEFI A, RASHCHIA F, MOGHADDAM J. Diagnostic pre-treatment procedure for simultaneous cyanide leaching of gold and silver from a refractory gold/silver ore [J]. Minerals Engineering, 2011, 24(15): 1703-1709.
[2] NANTHAKUMAR B, PICKLES C A, KELEBEK S. Microwave pretreatment of a double refractory gold ore [J]. Minerals Engineering, 2007, 20(11): 1109-1119.
[3] CELEPA O, ALPA  PAKTUNCB D, THIBAULT Y. Implementation of sodium hydroxide pretreatment for refractory antimonial gold and silver ores [J]. Hydrometallurgy, 2011, 108(1-2): 109-114.
PAKTUNCB D, THIBAULT Y. Implementation of sodium hydroxide pretreatment for refractory antimonial gold and silver ores [J]. Hydrometallurgy, 2011, 108(1-2): 109-114.
[4] MA S J, LUO W J, MO W, SU X J, LIU P, YANG J L. Removal of arsenic and sulfur from a refractory gold concentrate by microwave heating [J]. Minerals Engineering, 2010, 23(11): 61-63.
[5] SOLTANI F, DARABI H, BADRI R, ZAMANKHAN P. Improved recovery of a low-grade refractory gold ore using flotation– preoxidation–cyanidation methods [J]. International Journal of Mining Science and Technology, 2014, 24(4): 537-542.
[6] KOMNITSAS C, POOLEY F D. Mineralogical characteristics and treatment of refractory gold ores [J]. Minerals Engineering, 1989, 2(4): 449-457.
[7] LABROOY S R, LINGE H G, WALKER G S. Review of gold extraction from ores [J]. Minerals Engineering, 1994, 7(10): 1213-1241.
[8] MICHELISA I D, AGOSTINO O, STEFANO U, FRANCESCO F, FRANCESCA B, FRANCESCO V. Roasting and chlorine leaching of gold-bearing refractory concentrate: Experimental and process analysis [J]. International Journal of Mining Science and Technology, 2013, 25(3): 709-715.
[9] VINALS J, JUAN E, ROCA A, CRUELLS M, CASADO J. Leaching of metallic silver with aqueous ozone [J]. Hydrometallurgy, 2005, 76(3-4): 225-232.
[10] RODRIGUEZ C, NAVA-ALONAO F, URIBE-SALAS A. Silver leaching from pyrargyrite oxidation by ozone in acid media [J]. Hydrometallurgy, 2014, 149: 168-176.
[11] CARRILLO-PEDROZA F R, NAVA-ALONSO F, URIBE-SALAS A. Cyanide oxidation by ozone in cyanidation tailings: Reaction kinetics [J]. Minerals Engineering, 2000, 13(5): 541-548.
[12] UKASIK M, HAVLIK T. Effect of selected parameters on tetrahedrite leaching by ozone [J]. Hydrometallurgy, 2005, 77(1-2): 139-145.
[13]  E, NAVA-ALONSO F, JARA J, LARA- VALENZUELAC C. Treatment of pyritic matrix gold–silver refractory ores by ozonization–cyanidation [J]. Minerals Engineering, 2006, 19(1): 56-61.
E, NAVA-ALONSO F, JARA J, LARA- VALENZUELAC C. Treatment of pyritic matrix gold–silver refractory ores by ozonization–cyanidation [J]. Minerals Engineering, 2006, 19(1): 56-61.
[14] LI Qing-cui, LI Deng-xin, QIAN Fang-jun. Pre-oxidation of high-sulfur and high-arsenic refractory gold concentrate by ozone and ferric ions in acidic media [J]. Hydrometallurgy, 2009, 97(1-2): 61-66.
[15] SAHOO P R, VENKATESH A S. Constraints of mineralogical characterization of gold ore: Implication for genesis, controls and evolution of gold from Kundarkocha gold deposit, eastern India [J]. Journal of Asian Earth Sciences, 2015, 97 (Part A): 136-149.
[16] WANG Fang-fang, ZHAO Yue-min, ZHANG Tao, DUAN Chen-long, WANG Li-zhang. Mineralogical analysis of dust collected from typical recycling line of waste printed circuit boards [J]. Waste Management, 2015, 43: 434-441. (in Chinese)
[17] QIN Hong-gen, SONG Bin-jie, LIN Chun-ying. The study on process mineralogy of a gold ore from Zhengyuan Gold Mine [J]. Gold, 2005, 26(12): 36-39. (in Chinese)
[18] WANG Ji-kun, LEI Ting. Treating low grade antimony ore by bath smelting-continuous fuming process [J]. Nonferrous Met, 2000, 52: 44-48. (in Chinese)
[19] ANDERSON C G. The metallurgy of antimony [J]. Chemie der Erde – Geochemistry, 2012, 72(S4): s3-s8.
[20] RASCHMAN P, SMINCAKOVA E. Kinetics of leaching of stibnite by mixed Na2S and NaOH solutions [J]. Hydrometallurgy, 2012, 113: 60-66.
[21] UBALDINI S, VEGLIO F, FORNARI P, ABBRUZZESE C. Process flow-sheet for gold and antimony recovery from stibnite [J]. Hydrometallurgy, 2000, 57: 187-199.
[22] YANG Jian-guang, WU Yong-tian. A hydrometallurgical process for the separation and recovery of antimony [J]. Hydrometallurgy, 2014, 143: 68-76.
[23] DU Xin-ling, TANG Chang-qing. Production technology of high-purity antimony white [J]. Liaoning Chem Ind, 2008, 36(12): 853-856. (in Chinese).
[24] TIAN Qing-hua, WANG Heng-li, XIN Yun-tao, LI Dong, GUO Xue-yi. Ozonation leaching of a complex sulfidic antimony ore in hydrochloric acid solution [J]. Hydrometallurgy, 2016, 159: 126-131.
[25] MARCEL P. Atlas d'Equilibres Electrochimiques [M]. Paris: Gauthier-Villars,1963: 400-406.
郭学益1,2,辛云涛1,2,王 浩1,2,田庆华1,2
1. 中南大学 冶金与环境学院,长沙 410083;
2. 中国有色金属工业协会 中国清洁冶金工程研究中心,长沙 410083
摘 要:针对复杂难处理金矿的矿物学特征和盐酸体系臭氧氧化预处理提锑开展研究。工艺矿物学研究表明,矿物中主要存在辉锑矿、毒砂、黄铁矿和脉石,67.42%的金被包裹在硫化物之中。开展盐酸体系中臭氧氧化提锑的研究,考察反应温度、液固比、盐酸浓度和搅拌速度等因素对提锑过程的影响,在优化实验条件下,锑的浸出率可达93.75%。经过臭氧氧化预处理过程,矿物中的锑得到有效回收,同时包裹在辉锑矿和部分黄铁矿中的金得到释放和暴露。
关键词:含锑难处理金矿;臭氧;预处理;矿物学特征;提锑
(Edited by Xiang-qun LI)
Foundation item: Project (51474257) supported by the National Natural Science Foundation of China; Project (2015zzts037) supported by the Postgraduate Research and Innovation Projects of Hunan province, China; Project (2015JC3005) supported by the Key Technology Research and Development Program of Hunan Province, China
Corresponding author: Qing-hua TIAN; Tel: +86-731-88877863; E-mail: qinghua@csu.edu.cn
DOI: 10.1016/S1003-6326(17)60213-9

