Trans. Nonferrous Met. Soc. China 23(2013) 538-542
Preparation of ultrafine rhenium powders by CVD hydrogen reduction of volatile rhenium oxides
Meng BAI, Zhi-hong LIU, Le-jun ZHOU, Zhi-yong LIU, Chuan-fu ZHANG
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 11 November 2011; accepted 3 May 2012
Abstract: A novel CVD process for the preparation of ultrafine rhenium powders was investigated using ammonium perrhenate as starting materials. In the process, volatile rhenium oxides, such as ReO4 and Re2O7, were vaporized under a controlled oxidizing atmosphere via the pyrolysis of ammonium perrhenate, and carried into reduction zone by carrier gas, and there reduced into rhenium powders by hydrogen gas. Thermodynamic calculations indicated that Re2O7 could be prevented from further decomposition through controlling the oxygen partial pressure higher than 10-1.248 Pa in the pyrolysis of ammonium perrhenate. This result was further validated via DSC-TGA analysis of ammonium perrhenate. The typical rhenium powders prepared by the CVD method proposed show irregular polyhedron morphology with particle size in the range of 100-800 nm and a D50 of 308 nm. The specific surface area and oxygen content were measured to be 4.37 m2/g and 0.45%, respectively.
Key words: ultrafine rhenium powders; ammonium perrhenate; chemical vapor decomposition; oxygen partial pressure
1 Introduction
Rhenium is a refractory metal that has unknown ductility to brittle transition temperature and a high modulus of elasticity. Components formed from rhenium can withstand repeated heating and cooling cycles without incurring mechanical damage. For these and other reasons, rhenium and its alloys are widely used in such areas as national defense, aerospace, electronics and petrochemistry [1-3]. Due to the melting point of rhenium up to 3180 °C, rhenium components are usually manufactured by powder metallurgy techniques using rhenium powder as raw material [4]. Therefore, the preparation of rhenium powders of excellent performance is a prerequisite in rhenium industry.
At present, there are two principal methods of preparing rhenium powders commercially: one is direct hydrogen reduction of ammonium perrhenate through gas-solid reactions [5-7]; while the other is electrolysis [8-10]. Both of them are simple and easy to implement in large scale. However, the rhenium powders made by these two methods have some drawbacks such as irregular morphology, broad size distribution, low tap density and poor fluidity, which results in the difficulty of manufacturing rhenium products via conventional powder metallurgy.
Plasma technique can prepare rhenium powders of spherical morphology and good fluidity, but it is extremely cost ineffective [11-13]. In addition, rhenium or its alloy coating can be obtained from rhenium halides by means of CVD method [14-16], but highly anticorrosive materials must be used for manufacturing the equipments used in the processes due to strong corrosiveness of rhenium halides.
In this work, a novel CVD process for preparing ultrafine rhenium powders using ammonium perrhenate as raw material was investigated. The process was carried out in a tube furnace including evaporation and reduction zones, and essentially consisted of evaporation-reduction of volatile rhenium oxides. In the evaporation zone, highly volatile rhenium oxides were generated via the pyrolysis of ammonium perrhenate under a controlled oxygen pressure, at which volatile rhenium(VII) oxide can be prevented from further decomposition into low valent rhenium oxides to volatilize. Gaseous rhenium oxides were carried out by mixed oxygen and nitrogen gases into the reduction zone, and there reduced into rhenium powders by hydrogen gas.
2 Experimental
2.1 Materials
Ammonium perrhenate used for the preparation of rhenium powders was obtained from Jiangxi Copper Company in China, and its purity was larger than 99.5%. The gases of oxygen, nitrogen and hydrogen used in the experiments were with purity of 99.999%.
2.2 Methods
As shown in Fig. 1, CVD reactor used for the experiment was a tube furnace composed of evaporation and reduction zones, which were connected through a hole in the partition wall, and controlled at 673.15 K and 1273.15 K, separately.
In the experiment, a measured amount of ammonium perrhenate in a quartz boat was placed in the evaporation zone, and pyrolyzed into volatile rhenium (VII) oxide at 673.15 K under a controlled oxygen pressure, at which volatile rhenium (VII) oxide, Re2O7, can be prevented from further decomposition into low valent rhenium oxides, ReO3 and ReO2, which are difficult to volatilize. The gaseous rhenium oxides that were carried by mixed oxygen and nitrogen gases went into the reduction zone, and in this zone, they were reduced into rhenium powders by hydrogen gas.
2.3 Characterizations
DSC-TGA analyses of ammonium perrhenate were carried out using a Q600 integrated thermal analyzer. The morphology of the rhenium powders was inspected by SEM using a JSM-6360LV scanning electron microscope. The structures of the samples were evaluated by XRD using a Rigaki-TTR Ⅲ X-ray diffractometer with Cu Kα (0.154184 nm) source. The particle size distributions of the rhenium powders were determined by using a Malvern Nano-ZS particle size analyzer. The oxygen content of the rhenium powders was measured by using a LECO TCH-600 N/O analyzer. Specific surface area (BET) was measured by using a quantachrome monosorb specific surface area analyzer.
3 Results and discussion
3.1 Theoretic analysis
In the experiment, when ammonium perrhenate is placed in the evaporation zone at 673 K under a mixed oxygen and nitrogen atmosphere, it will decompose thermally into gaseous Re2O7 volatilizing according to following equation:
2NH4ReO4+(3/2)O2=Re2O7(g)+4H2O(g)+N2(g) (1)
Rhenium is a kind of multi-valence metal and there are four rhenium oxides present in Re-O system: ReO4, Re2O7, ReO3 and ReO2, the vapor pressures of which at different temperatures are shown in Fig. 2. Re2O7 obtained by thermal decomposition of ammonium perrhenate may be further oxidized into ReO4 or deoxidized into ReO3, ReO2 and even Re at high temperatures.
Re2O7+1/2O2(g)=2ReO4 (2)
Re2O7=2ReO3+(1/2)O2(g) (bellow 800 K) (3)
Re2O7=2ReO2+(3/2)O2(g) (above 800 K) (4)
ReO3=2ReO2+(1/2)O2(g) (5)
2ReO2=2Re+2O2(g) (6)
From Fig. 2, it can be seen that high valent rhenium oxides, ReO4 and Re2O7, with the boiling points of 493 K and 635.4 K, respectively, are easier to volatilize than low valent rhenium oxides, ReO3 and ReO2, with the boiling points of 887 K and 1636 K, individually. Therefore, reactions (3) to (6) should be prevented from taking place by controlling oxygen potential of the system, in order to transfer rhenium oxides in gaseous form at a desired temperature into the reduction zone.
Figure 3 shows the oxygen potential diagram of Re-O system, which was calculated by using HSC Chemistry 5.0 software. From Fig. 3, it can be seen that Re2O7 can be prevented from deoxidizing into ReO3 or ReO2 by controlling the oxygen pressure above 1013.25 Pa in the experimental temperature range of 673.15- 1273.15 K.
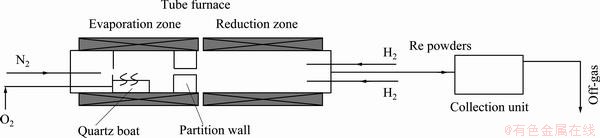
Fig. 1 CVD apparatus for preparing ultra-fine rhenium powders
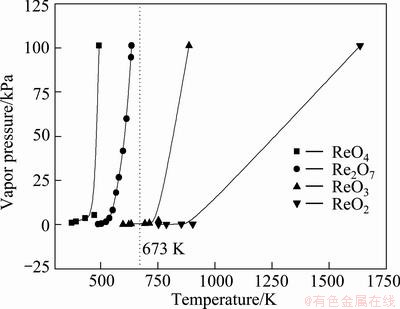
Fig. 2 Vapor pressure of rhenium oxides at different temperatures [17,18]
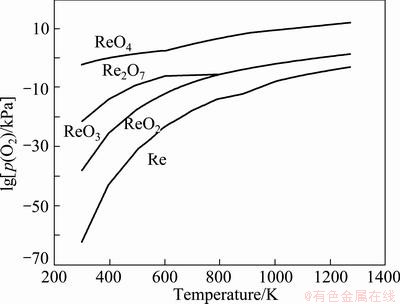
Fig. 3 Oxygen potential diagram of Re-O system
In the reduction zone, ReO4 and Re2O7 are reduced at 1273.15 K by H2 introduced from the right side of the tube furnace.
ReO4(g)+4H2(g)=Re+4H2O(g) (7)
Re2O7(g)+7H2(g)=2Re+7H2O(g) (8)
3.2 DSC-TGA analysis of ammonium perrhenate in air and nitrogen gas
The DSC-TGA curves of NH4ReO7 obtained in air and nitrogen gas at heating rate of 10 K/min are shown in Fig. 4 and Fig. 5, respectively. Figure 4 reveals that under oxygen atmosphere (in air), NH4ReO7 pyrolyzed wholly into Re2O7 volatilizing at about 673 K, in according with theoretic analysis mentioned in section 3.1. From Fig. 5, it can be seen that under inert atmosphere (in N2), the products of NH4ReO7 decomposition volatilize wholly till up to 1373 K, due to volatile Re2O7 further decomposing into low valent ReO3 and/or ReO2, which are difficult to volatilize, as shown in Fig. 2.
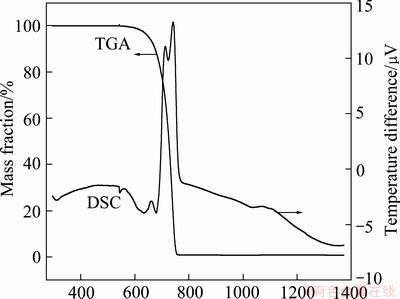
Fig. 4 DSC-TGA curves of NH4ReO4 in air at heating rate of 10 K/min
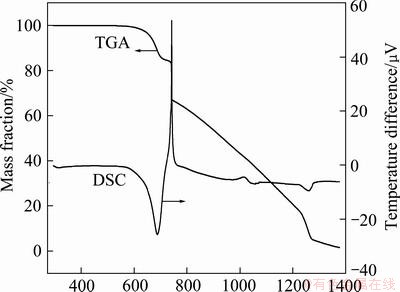
Fig. 5 DSC-TGA curves of NH4ReO4 in nitrogen at heating rate of 10 K/min
3.3 Effects of oxygen flow on residue of NH4ReO4 pyrolyzing
The experiments were carried out in the CVD apparatus shown in Fig. 1. Under the conditions of temperature 673 K and nitrogen flow 60 L/h, 20 g NH4ReO4 sample in the quartz boat placed in the evaporation zone was pyrolyzed for 1 h at different oxygen flow rates. The residual mass of NH4ReO4 pyrolyzed in the boat changed with the variation of oxygen flow rates, as shown in Table 1. Figure 6 shows the XRD patterns of residues of NH4ReO4 pyrolyzing at oxygen flow rates of 40 and 20 mL/min under the conditions mentioned above.
Table 1 Residue mass of 20 g NH4ReO4 pyrolyzing at different oxygen flow rates
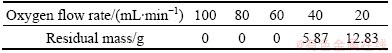
From Table 1 and Fig. 6, it can be seen that the oxygen pressure has a great effect on the decomposition of the products in the process of NH4ReO4 pyrolyzing. Under the experimental conditions, NH4ReO4 was decomposed into Re2O7 volatilizing wholly at oxygen flow rates greater than 60 mL/min; while NH4ReO4 was partially decomposed into ReO3 (JCPDS 65—9764) or the mixture of ReO3 and ReO2 (JCPDS 17—0600) at oxygen flow rates of 40 mL/min and 20 mL/min, respectively.
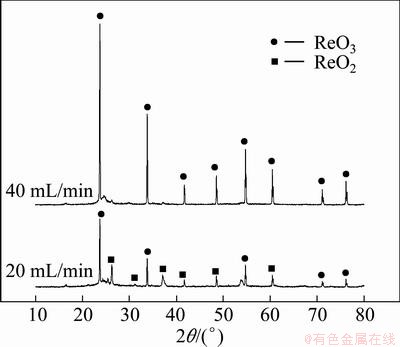
Fig. 6 XRD patterns of residues of NH4ReO4 pyrolyzing at oxygen flow rates of 40 and 20 mL/min
3.4 Characterization of typical rhenium powders prepared
Rhenium powders were prepared by using the CVD apparatus shown in Fig. 1 under the conditions as follows: nitrogen gas flow rate 60 L/h, oxygen gas flow rate 60 mL/min, temperature of evaporation zone 673 K, surplus coefficient of hydrogen gas 6, and temperature of reduction zone 1273 K. The rhenium powders prepared under the conditions mentioned above were characterized by XRD, SEM, size distribution, specific surface area (BET) and oxygen content analyses.
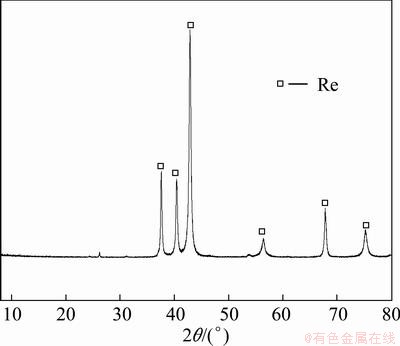
Fig. 7 XRD pattern of typical rhenium powders prepared by CVD method
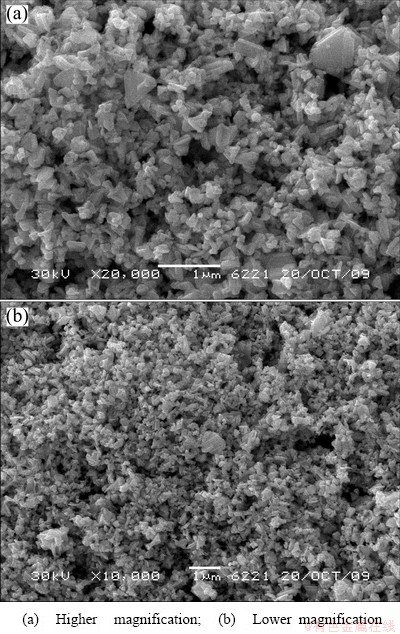
Fig. 8 SEM images of typical rhenium powders prepared by CVD method
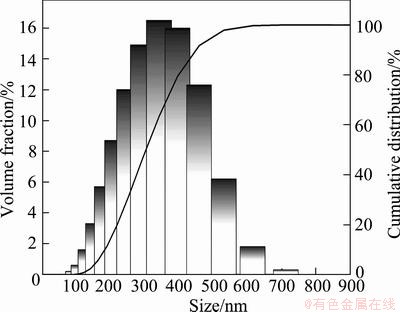
Fig. 9 Particle size distribution of rhenium powders prepared by CVD method
As shown in Fig. 7, XRD result demonstrated that the powders prepared by the CVD method proposed were in the form of metallic rhenium (JCPDS 05—0702). From the SEM photographs and the particle size distribution results shown in Fig. 8 and Fig. 9, separately, it can be seen that the rhenium powders have irregular polyhedron morphology with particle sizes in the range of 100-800 nm and a D50 of 308 nm. The specific surface area and oxygen content of the powders were measured to be 4.37 m2/g and 0.45%, respectively.
4 Conclusions
1) A novel CVD method of preparing ultrafine rhenium powders was proposed and investigated. It uses ammonium perrhenate as starting material and essentially consists of evaporation-reduction of highly volatile rhenium oxides (Re2O7).
2) In the preparation process, to convey rhenium oxides produced through thermal decomposition of ammonium perrhenate, in gaseous form at a desired temperature into reduction zone, the further decomposition of volatile Re2O7 into low valent rhenium oxides of difficulty to volatile, such as ReO3 and ReO2, should be prevented from.
3) Thermodynamic analysis and experiments demonstrated that the further decomposition of Re2O7 into ReO3 and ReO2 can be prevented from by controlling the oxygen pressure of the process.
4) The typical rhenium powders prepared by the CVD method proposed have irregular polyhedron morphology with particles sizes in the range of 100-800 nm and a narrow size distribution.
References
[1] Naor A, Eliaz N, Gileadi E, Taylor R. Properties and applications of rhenium and its alloys [J]. The AMMTIAC Quarterly, 2010, 5(1): 11-14.
[2] LOU Zhe-ning, XIONG Ying, SONG Jun-jun, SHAN Wei-jun, HAN Guang-xi, XING Zhi-qiang, KONG Yu-xia. Kinetics and mechanism of Re(VII) extraction and separation from Mo(VI) with trialkyl amine [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 10-14.
[3] Busby J T, Leonard K J, ZINKLE S J. Radiation-damge in molybdenum-rhenium alloys for space reactor applications [J]. Journal of Nuclear Materials, 2007, 366: 388-406.
[4] Trybus C L, Wang C M, Pandheeradi M, Meglio C A. Powder metallurgical processing of rhenium [J]. Advanced Materials & Processes, 2002, 160(12): 23-26.
[5] Stefan L, Helmut A. Hydrogen as a reducing agent: State-of-the-art science and technology [J]. JOM, 2007, 59(6): 20-16.
[6] Mnnheim R L, Garin J L. Microstructural characterization of rhenium powder obtained at various temperatures [J]. Advanced Powder Technology, 2003, 416(4): 273-282.
[7] SHEN You-yuan, YI Xiao-ming, LIAO Bin-bin. A preparation method of high purity rhenium powders: CN02114247.5 [P]. 2002-07-08. (in Chinese)
[8] Schrebler R, Cury P. Electrochemical and nano- electrogravimetric studies of the nucleation and growth mechanisms of rhenium on polycrystalline gold electrode [J]. Electrochimica Acta, 2001, 46: 4309-4318.
[9] Schrebler R, Merino M, Cury P, Romo M, Cordova R, Gomez H, Dalchiele E A. Electrodeposition of Cu-Re alloy thin films [J]. Thin Soild Films, 2001, 388: 201-207.
[10] Naor A, Eliaz N, Gileadi E. Electrodeposition of rhenium-nickel alloy from aqueous solutions [J]. Electrochimica Acta, 2009, 54(25): 6028-6035.
[11] JUREWICZ J W, GUO Jia-yin. Process for plasma synthesis of rhenium nano and micro powders, and for coating and near net shape deposits thereof and apparatus therefore: US: 2005/0211018A1 [P]. 2005-09-29.
[12] LEONHAROT T, HAMISTER M, MOURE N, DOWNS T. Advancements in powder metallurgy rhenium [C]// Kubel. Advances in Powder Metallurgy and Particulate Materials. New Orleans: Metal Powder Industries Federation, 2001: 408-422.
[13] Leonhardt T, Hickman R. Consolidation methods for spherical rhenium and rhenium alloys [J]. Powder Metallurgy, 2003, 46(2): 148-153.
[14] Goncharov O Y, Faizullin R R, Shadrin M G, Kanunnikov M F. Chemical vapor deposition of Mo, Re, and Ta films [J]. Inorganic Materials, 1999, 35(10): 985-987.
[15] Hu C Y, Chen S, Yang J M, Deng D G, Gao Y Q, Yin Y M, Wang Y, Li J H. Diffusion study in interface of iridium/rhenium composite prepared by CVD [J]. Rare Metal Materials and Engineering, 2003, 32(10): 796-798. (in Chinese)
[16] Li Jing-hua, Hu Chang-yi, Gao Yi-qun. Study of rhenium tube prepared by chemical vapour deposition [J]. Aerospace Materials & Technology, 2001(4): 54-56. (in Chinese)
[17] Frenkel M, Kabo G J, Marsh K N, Roganov G N, Wilhoit R C. Thermodynamics of organic compounds in the gas state [M]. Vol: 1-2. Texas: TRC, 1994.
[18] ZHOU Ling-zhi, CHEN Shao-chun. Extractive metallurgy of scattered metals [M]. Beijing: Metallurgical Industry Press, 2008: 141-150. (in Chinese).
采用CVD法还原挥发性铼的氧化物制备超细铼粉
白 猛,刘志宏,周乐君,刘智勇,张传福
中南大学 冶金科学与工程学院,长沙 410083
摘 要:研究了一种以高铼酸铵为原料,采用化学气相沉积(CVD)制备超细铼粉的新方法。通过控制氧分压,使得NH4ReO7分解为具有挥发性的ReO4、Re2O7,再采用载气将其输运至还原区,经氢气还原生成超细铼粉。热力学计算表明,在NH4ReO7分解过程中,控制氧分压高于10-1.248 Pa时,Re2O7将不会分解为低价氧化物,DSC-TGA分析结果也证实了这一点。采用该方法制备的铼粉,粒度为100~800 nm,D50为308 nm,比表面积为4.37 m2/g,氧含量为0.45%。
关键词:超细铼粉;高铼酸铵;化学气相沉积;氧分压
(Edited by Hua YANG)
Corresponding author: Zhi-hong LIU; Tel: +86-731-88830478; E-mail: zhliu@csu.edu.cn
DOI: 10.1016/S1003-6326(13)62496-6