新型1-(2-羟苯基)己-2-烯-1-酮肟浮选捕收剂对孔雀石的浮选性能及吸附机理
来源期刊:中国有色金属学报(英文版)2020年第10期
论文作者:李方旭 周晓彤 林日孝
文章页码:2792 - 2801
关键词:孔雀石;浮选;捕收剂;肟;吸附机理
Key words:malachite; flotation; collector; oxime; adsorption mechanism
摘 要:以2-羟基苯乙酮和丁醛为原料合成一种新型1-(2-羟苯基)己-2-烯-1-酮肟(HPHO)捕收剂。通过浮选试验、zeta电位、傅里叶变换红外光谱(FTIR)和X射线光电子能谱(XPS)分析技术研究HPHO对孔雀石的浮选性能和吸附机理。结果表明,与苯甲羟肟酸(BA)、1-(2-羟苯基)乙-1-酮肟(HPEO)和异丁基黄药(SIBX)相比,HPHO对孔雀石具有更好的捕收性能且无需额外添加调整剂Na2S和起泡剂甲基异丁基甲醇(MIBC)。Zeta电位结果发 现,HPHO可能通过化学作用吸附在孔雀石表面。 FTIR和XPS结果表明,HPHO通过C=C、—OH和N—OH基团与孔雀石表面的铜物种键合形成Cu-肟配合物。
Abstract: A novel collector 1-(2-hydroxyphenyl)hex-2-en-1-one oxime (HPHO) was synthesized from 2-hydroxy acetophenone and butyraldehyde. Its flotation performance and adsorption mechanism to malachite were investigated by flotation test, zeta potential, Fourier transform infrared (FTIR) and X-ray photoelectron spectroscopy (XPS) analysis techniques. Compared with benzohydroxamic acid (BA), 1-(2-hydroxyphenyl)ethan-1-one oxime (HPEO) and sodium isobutyl xanthate (SIBX), HPHO exhibited excellent collecting power to malachite without additional reagents, such as Na2S regulator and methyl isobutyl carbinol (MIBC) frother. Results of zeta potential indicated that HPHO was coated on malachite surfaces through a chemisorption process. FTIR and XPS data gave clear evidence for the formation of Cu-oxime complex on malachite surfaces after HPHO adsorption through the linkage between C=C, —OH, N—OH group and Cu species.
Trans. Nonferrous Met. Soc. China 30(2020) 2792-2801
Fang-xu LI1,2,3,4, Xiao-tong ZHOU1,2,3,4, Ri-xiao LIN1,2,3,4
1. Guangdong Academy of Sciences, Guangzhou 510650, China;
2. Guangdong Institute of Resources Comprehensive Utilization, Guangzhou 510650, China;
3. State Key Laboratory of Rare Metals Separation and Comprehensive Utilization, Guangzhou 510650, China;
4. Guangdong Provincial Key Laboratory of Development and Comprehensive Utilization of Mineral Resources, Guangzhou 510650, China
Received 15 January 2020; accepted 15 July 2020
Abstract: A novel collector 1-(2-hydroxyphenyl)hex-2-en-1-one oxime (HPHO) was synthesized from 2-hydroxy acetophenone and butyraldehyde. Its flotation performance and adsorption mechanism to malachite were investigated by flotation test, zeta potential, Fourier transform infrared (FTIR) and X-ray photoelectron spectroscopy (XPS) analysis techniques. Compared with benzohydroxamic acid (BA), 1-(2-hydroxyphenyl)ethan-1-one oxime (HPEO) and sodium isobutyl xanthate (SIBX), HPHO exhibited excellent collecting power to malachite without additional reagents, such as Na2S regulator and methyl isobutyl carbinol (MIBC) frother. Results of zeta potential indicated that HPHO was coated on malachite surfaces through a chemisorption process. FTIR and XPS data gave clear evidence for the formation of Cu-oxime complex on malachite surfaces after HPHO adsorption through the linkage between C=C, —OH, N—OH group and Cu species.
Key words: malachite; flotation; collector; oxime; adsorption mechanism
1 Introduction
Surfactant is recognized as one of the most important factors in the modification of materials’ surface and has been used in a variety of practical applications including material protection, minerals engineering and oil-water separation [1-3]. In its application to mineral engineering (known as a collector), the main function of a surfactant is to create a hydrophobic surface on target minerals selectively so that the hydrophobized mineral can be floated out and separated from gangue minerals [4].
Malachite is a kind of typical copper oxide mineral and can serve as a new source of copper to meet the huge market demand. Currently, two methods of sulfide flotation and direct flotation are usually employed in flotation of malachite. As for sulfide flotation, sodium isobutyl xanthate (SIBX) does not perform well to malachite, unless it is activated by Na2S. In addition, sulphidization of copper oxide ore is hard to control due to complex ore properties [5]. As to direct flotation, fatty acids, fatty amines, sulfonates and phosphonates reveal poor selectivity to separate malachite from calcite [6-8]. Hydroxamic acids such as benzo- hydroxamic acid (BA) and octyl hydroxamic acid show satisfying flotation performance to malachite [9]. Similar to hydroxamic acids, oxime collectors have not received enough attention during the past two decades.
The C=N—OH group of oxime molecule has a long pair electron and is always recognized as an electron-donor for metal ion. And its corresponding oximes have been proved to be effective collectors for copper oxide ore because of their selective complexation with copper ions [10]. The model of Cu(II)-salicylaldoxime complex is proposed as six-membered ring structure according to Cecile’s work (see Scheme 1) [11]. Salicylaldoxime is a typical oxime collector widely studied in copper oxide ore, cassiterite and wolframite flotation [10-13].
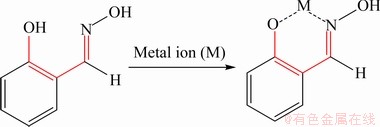
Scheme 1 Proposed mechanism for salicylaldoxime reacting with metal ion
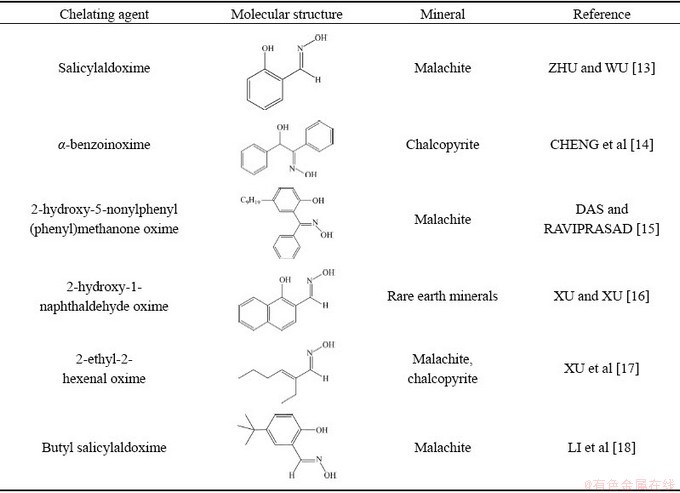
In order to develop new collectors with superior flotation performance, flotation scientists and engineers have paid a lot of attention to the modification of traditional oximes. α-benzoinoxime exhibits good collecting properties for the sulfide minerals [14]. 2-hydroxy-5-nonylphenyl (phenyl) methanone oxime shows better flotation performance than conventional xanthate collectors in terms of the recovery of copper from the Rakha ore [15]. 2-hydroxy-1-naphthaldehyde oxime is selected as a collector for rare earth ores (REO) [16]. 2-ethyl-2-hexenal oxime, as a non- aromatic oxime, can recover malachite and chalcopyrite minerals in the alkaline environment [17]. Tert-butyl salicylaldoxime shows powerful collecting ability to malachite [18]. Based on the above studies, modification of hydrophobic group of oximes may be a good choice for developing novel collectors for copper oxide ore (see Table 1).
Aldol condenzation is a convenient method to acquire new aldehydes or ketones, which can be easily obtained without the use of precious metal catalysis and harsh reaction conditions [19]. Subsequently, ketone oximes can be synthesized by the dehydration of product of aldol condensation with hydroxylamine hydrochloride [20]. To the best of our knowledge, 1-(2-hydroxyphenyl)hex-2-en-1- one oxime (HPHO) for flotation of malachite has been hardly reported in previous literatures [13-18].
Table 1 Chemical structure of several collectors in literatures
In this work, a novel collector HPHO was synthesized through aldol condenzation and ammoximation reaction. Its chemical structure was characterized by FTIR, LC-MS and NMR. The flotation performance of HPHO to malachite was investigated by micro and batch flotation. Moreover, the adsorption mechanism of HPHO to malachite was discussed by zeta potential, FTIR and XPS analysis. As is comprehensively revealed below, this integrated approach provides a detailed image of HPHO molecular behavior on the malachite/ water interface.
2 Experimental
2.1 Materials
The X-ray diffraction (XRD) pattern of malachite is presented in Fig. 1. The result of chemical analysis showed that malachite was 97% pure. Additionally, 38-75 μm fractions of malachite were used in micro-flotation experiment. Fractions with sizes less than 5 μm were prepared for zeta potential, FTIR and XPS measurements. Copper oxide ore used in the batch flotation came from Yunnan Province, China. The most abundant copper oxide mineral was malachite (2.89%). The major gangue mineral in the ore was quartz. 1-(2-hydroxyphenyl)ethan-1-one oxime(HPEO) was prepared by Hyodo’s method [21]. SIBX and BA were obtained from Zhuzhou Flotation Reagents & Chemicals Co., Ltd. Prior to the flotation, the collector was dispersed through NaOH aqueous solution under ultrasound condition. HPHO was synthesized in our laboratory, whose synthesis route is listed in Scheme 2.
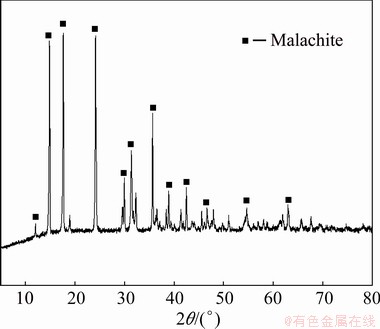
Fig. 1 XRD pattern of malachite
2.2 Flotation test
A micro-flotation experiment was conducted by operating an XFG II flotation machine with an effective cell volume of 40 mL. The impeller speed of flotation machine was l650 r/min. Firstly, malachite was dispersed in water for 2 min. After the pH adjustment, a collector was introduced and subsequently conditioned for 2 min. Finally, the conditioned slurry was floated for 5 min. The flotation results of malachite were calculated by the dry masses of the concentrates and tails.
A batch-flotation experiment was conducted by operating an XFG II flotation machine with an effective cell volume of 500 mL. The impeller speed of flotation machine was 1992 r/min. Initially, the copper oxide ore was ground to be less than 74 μm (>80 wt.%) in an XMB-70 rod mill. The pH of copper oxide ore for batch flotation was adjusted to be around 8.0. Before flotation, a conditioning time of 3 min was allowed after the addition of the collector. The conditioned slurry was floated for 6 min. Finally, the concentrates and tailings were dried and weighed. And the amount of Cu was detected through a chemical analysis.
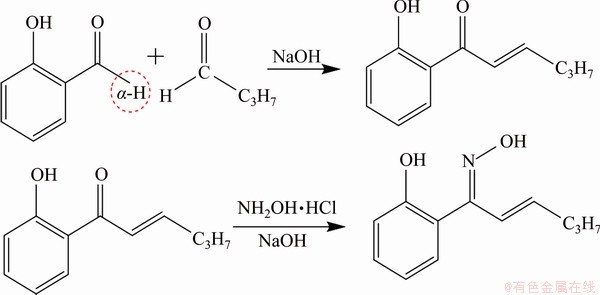
Scheme 2 Synthetic route of HPHO
2.3 Zeta potential measurement
The zeta potential of malachite was detected by using a Coulter Delsa 440 SX zeta potential and particle size analyzer. Next, malachite samples with sizes less than 5 μm were added to 0.01 mol/L KNO3 solution. And the pH levels of the suspension were adjusted using diluted NaOH or HNO3 solutions. The zeta potentials of the malachite before and after HPHO treatment were determined and recorded. Five independent measurements of each sample were conducted with the average of zeta potential and the standard deviation was calculated.
2.4 FTIR measurement
FTIR measurements were carried out on a Nicolet model Nexus 670 instrument. Four samples, including HPHO, malachite, Cu(II)-HPHO complex and HPHO-malachite products, were recorded based on the measurements. The Cu(II)-HPHO complex and HPHO-malachite product were prepared by the following method. Cu(II) solution and malachite were slowly added to HPHO- methanol-water mixture at 298 K with vigorously stirring. After 2 h, the precipitate and modified malachite were washed with methanol-water (1:1, volume ratio) mixed solution several times to eliminate any residual surfactant and later dried with a vacuum.
2.5 XPS analysis
An XPS analysis was done on a Thermo Scientific ESCALAB 250Xi instrument with an Al Kα X-ray source and operated at 200 W with a pass energy of 20 eV. All the samples were analyzed under 1.33×10-7-1.33×10-6 Pa and the take-off angle was 45°. Malachite, HPHO, Cu(II)-HPHO complex and HPHO-malachite products were picked out to reveal the adsorption mechanism. The data were carefully collected and treated with the help of Thermo Scientific Advantage 4.52 software.
3 Results
3.1 Preparation and characterization of HPHO
HPHO was prepared by utilizing 2-hydroxy acetophenone, butyraldehyde and NH2OH·HCl as the starting materials. Firstly, 2-hydroxy acetophenone and butyraldehyde were added to methanol (50 mL) by using 10% NaOH (5 mL) as the catalyst. And then the solution was stirred at 60 °C for 6 h. Next, NH2OH·HCl (7.65 g, 0.11 mol) in H2O (50 mL) was added to a mixture of 1-(2-hydroxyphenyl)hex-2-en-1-one without any separation and purification. Thereafter, NaOH was added to adjust the pH of solution until reaching 7-8. Then, they reacted at 50 °C for 4 h. Finally, the brown crude mixture was obtained after removing the solvent. Product characterization: 1-(2-hydroxyphenyl)hex-2-en-1-one oxime (HPHO), a light brown solid, yield 72.5%. FTIR(KBr): (3361 (—OH), 3067 (C—H), 2971 and 2905 (CH3— and —CH2—), 1328, 1464 and 1605 (C=C), 1681 (C=N) cm-1); MS(ESI+): calculated for C12H15NO2 205.11; found 206.12[M+H]; 1HNMR (300 MHz, [D6]DMSO): δ=11.61 (s, 1H, NOH), 8.58 (s, 1H, NH), 6.8-7.8 (m, 4H, CH), 5.5 (s, 1H, CH), 4.5 (s, 1H, CH), 2.35-2.39 (m, 2H, CH2), 1.50-1.55 (m, 2H, CH2), 0.90 (t, 3H, CH3); 13CNMR (300 MHz, [D6]DMSO): δ=157.3 (C=N), 157.1 (C—OH), 136.2, 130.3, 127.8, 119.3, 119.0, 117.7, 116.4 (C=C), 17.9, 13.9 (CH2), 10.9 (CH3).
3.2 Flotation behavior
Micro-flotation tests were performed with HPHO, HPEO, BA and SIBX. Figure 2(a) shows flotation performance of malachite at different pH values and 0.98 mmol/L HPHO. This reveals that the recovery of malachite increases at varying pH from 3 to 8. When the pH of pulp is higher than 8, the recovery of malachite decreases insignificantly until pH around 11. At a pH of 8, 98.1% of malachite is floated out. Figure 2(b) presents flotation recovery of malachite at HPHO concentrations ranging from 0.15 to 1.22 mmol/L, which shows the recovery of malachite increases rapidly at varying concentration from of 0.15 to 0.39 mmol/L. When the collector concentration is more than 0.73 mmol/L, the recovery increases slowly.
Table 2 and Fig. 3 present the flotation recoveries of malachite with the four collectors in the presence and absence of MIBC or Na2S. The results in Fig. 3 show that under pH 7-10, SIBX can float out more than 80% malachite in the presence of MIBC and Na2S. BA shows moderate recovery to malachite with the help of MIBC. HPEO reveals weak collecting ability in the case of MIBC. And less than 50% malachite is recovered at pH 3-12. Compared with SIBX, HPEO and BA, HPHO achieves superior malachite flotation recoveries under pH 8-12. Based on results of Table 2, it is clear that without the help of MIBC and Na2S, SIBX, HPEO and BA show poor collection capacity to malachite. As a matter of fact, less than 37% malachite is floated out in the absence of MIBC or Na2S. Whereas, HPHO achieves a satisfactory flotation performance without MIBC and Na2S. The batch flotation experiment shows that without desliming treatment, 0.98 mmol/L HPHO upgrades 4.13% Cu with 85.15% recovery.
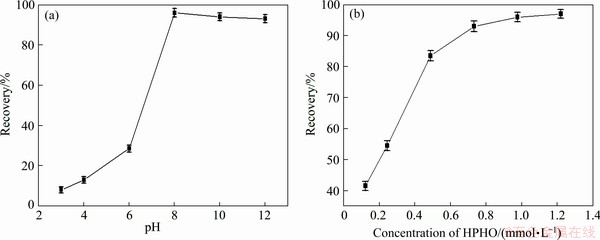
Fig. 2 Flotation recovery of malachite as function of pH value and concentration of HPHO
Table 2 Flotation results without or with MIBC and Na2S
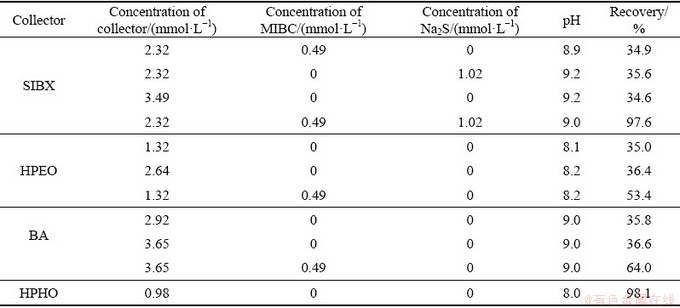
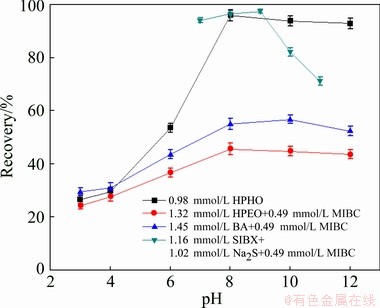
Fig. 3 Flotation recovery of malachite with different collectors
3.3 Zeta potential, FTIR and XPS spectra
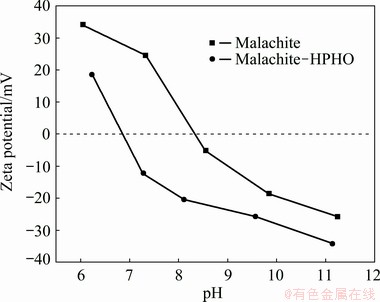
Fig. 4 Zeta potential of malachite as function of pH with or without HPHO treatment
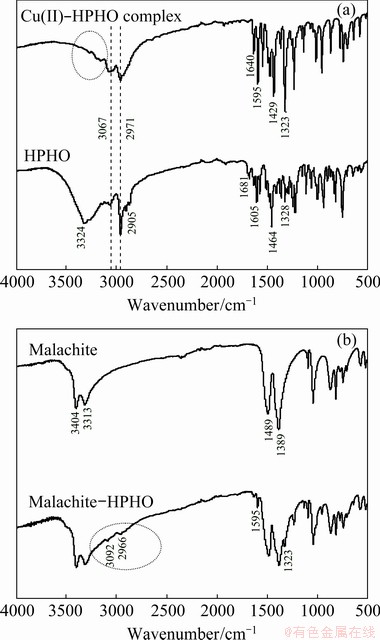
Fig. 5 FTIR spectra of HPHO and its Cu complex (a) and malachite before and after HPHO adsorption (b)
The zeta potential of malachite as a function of the pH value with or without HPHO treatment is shown in Fig. 4. The isoelectric point (IEP) of malachite stands at approximately pH 8.3 in 0.01 mol/L KNO3 solution, which is in agreement with our previous work [22]. The results indicate that the zeta potential of malachite generally decreases after being treated with HPHO. And IEP of malachite moves to 6.8.
The FTIR results of malachite, HPHO, Cu(II)-HPHO complex and malachite before and after HPHO adsorption are shown in Fig. 5. The characteristic peaks of HPHO, which are related to —OH, C=N, benzene ring, —CH3 and —CH2— groups, are found in Fig. 5. The stretching vibration of —O—H is found at 3324 cm-1. The stretching vibration of C—H on benzene ring is traced at around 3067 cm-1. The vibration of —CH3 and —CH2— can be seen at 2971 and 2905 cm-1, respectively. The stretching vibration of benzene ring or C=C is detected at 1328, 1464 and 1605 cm-1. The stretching vibration of C=N stands at 1681 cm-1 [23]. As to Cu(II)-HPHO complex, the peaks around 3067 and 2971 cm-1 owe to C—H, —CH2— and —CH3 groups; the peaks at 1323, 1429 and 1595 cm-1 are related to benzene ring, C=C and C=N groups, respectively. It is clear that the peak around 3324 cm-1 is weakened after reaction. Peaks of C=N and —OH bonds move to low frequency(red shift) [24]. The characteristic peaks of malachite are detected around 1489, 1389, 3404 and 3313 cm-1. After HPHO interaction, the peaks around 1323, 1595, 2966 and 3092 cm-1 are traced on the malachite surface, which are similar to FTIR of Cu(II)-HPHO complex.
The XPS results of malachite, Cu(II)-HPHO complex and HPHO-malachite products are shown in Fig. 6. Figure 6(a) presents the survey scan of malachite before and after HPHO adsorption. It indicates that the peak of N 1s is found on malachite surface. The relative intensity of the peak of C 1s increases after HPHO treatment as well. In order to find more data for adsorption and reaction mechanism, high-resolution XPS spectra of Cu 2p and O 1s are presented in Figs. 6(b, c), respectively. The Cu 2p3/2 and Cu 2p1/2 XPS peaks of malachite are found at around 936.3 and 955.9 eV, respectively. The shake-up peaks of Cu(II) species are traced at around 938 to 946 eV and 960 to 967 eV [22]. The Cu 2p3/2 and Cu 2p1/2 XPS peaks of Cu(II)-HPHO complex are detected at around 932.8, 934.3, 935.2, 952.1, 954.3 and 955.2 eV. Cu 2p3/2 and 2p1/2 peaks of HPHO-malachite prodcuts are observed around 932.9, 934.4, 935.3, 952.2, 954.4 and 955.5 eV. As for O 1s, the results in Fig. 6(c) illustrate the O 1s XPS peaks of malachite are made up of two components, 531.6 eV for CO32-, and 533.3 eV for —OH. After HPHO adsorption, the peak at around 533.3 eV is weakened. And the XPS peaks for C—O—Cu and N—O—Cu bonds are found at 531.3 and 533.1 eV.
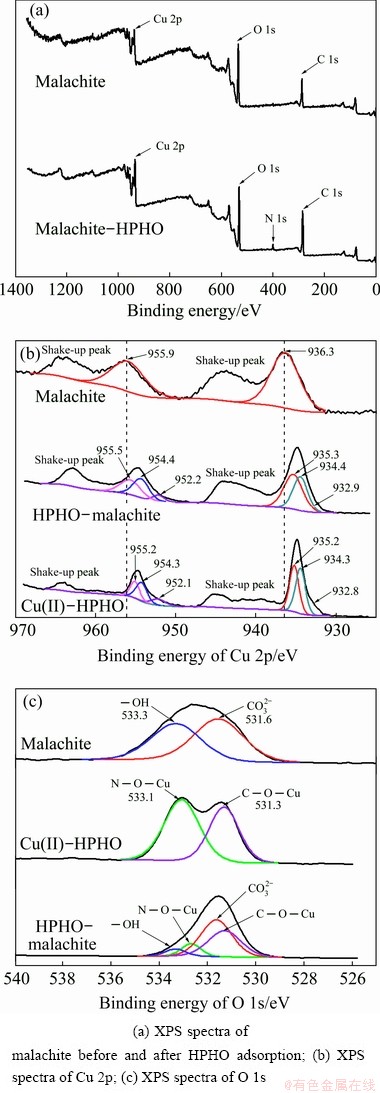
Fig. 6 XPS spectra of Cu(II)-HPHO complex, malachite before and after treating with HPHO
4 Discussion
Micro-flotation and batch experiments showed that HPHO was a useful collector to malachite. HPHO recovered 98.1% malachite at a pH of 8.0. Compared with SIBX, HPEO and BA, HPHO showed more powerful collection ability without Na2S and MIBC (see Fig. 3 and Table 2). It seemed that reasonable modification of molecular conduced oximes to more effectively change the hydrophobic properties of mineral (see Fig. 7). At the same time, long-chain collectors (HPHO) had better foaming properties than short-chain ones (HPEO and SIBX), which might be ascribed to the smaller critical micelle concentration (CMC) of long carbon HPHO [25]. The results of batch flotation experiment showed the concentrate (one rough stages) from the mine site assayed about 0.83% Cu. And the copper concentrate grade and recovery were 4.13% and 85.15%, respectively. It is not hard to see that HPHO has a huge potential to deal with low-grade copper ores in minerals engineering.
The result of zeta potentials gave a clear change of malachite before and after HPHO adsorption. In the presence of HPHO, the zeta potentials of malachite moved to more negative values, even at pH>8.3. Under base conditions, the mineral surface was negative. The anionized collector must overcome electrostatic repulsion, and then adsorbed on malachite surface. Thus, it was more reasonable to suggest a chemisorption process on malachite surface. The findings of zeta potentials were consistent with other oxime on malachite [18].
The results in Fig. 5 showed that the intensity of —OH in the Cu(II)-HPHO complex was weaker than that in HPHO, and a red shift phenomenon of C=C group was observed. This implied that the Cu(II) species donated partial d-orbital electrons to the C, N and O atoms in HPHO. Thereafter, C—O—Cu, N—O—Cu and d-pπ bonds might form between Cu(II), C=N—OH and C=C group. The FTIR spectra of malachite-HPHO products showed that the peak similar to Cu(II)-HPHO complex was traced on malachite after HPHO adsorption, further implying a chemisorption of HPHO on malachite surface. The results of XPS analysis offered promising evidence that HPHO reacted with Cu(II) active species on malachite. The N 1s XPS peak and the increase of relative intensity of C 1s on malachite indicated that numerous HPHO was coated on mineral surface. Moreover, the bond energy of Cu 2p can be clearly shifted after HPHO modification. The increase or decrease of the binding energy could reflect a change in the chemical environment of the atoms [25,26]. Generally speaking, the binding energy of an atom increases, which means that the atom contributes electron; whereas the binding energy of the atom decreases, which hints that the atom accepts external electron [27,28]. The binding energy of Cu 2p3/2 and Cu 2p1/2 of malachite-HPHO products or Cu(II)-HPHO complex decreased, which indicated that copper atom received electron from electronegative HPHO, and —OH, C=C and C=N—OH were the only groups in the HPHO molecule with lone pair electrons. Therefore, it can be concluded that copper ions bond with HPHO through d-pπ, N—O—Cu and C—O—Cu linkages. The inspection of the O 1s XPS was very important to examine the chemical changes on malachite after HPHO modification. The decrease of the relative peak ratio of —OH with respect to CO32- after HPHO treatment indicated the —OH groups on malachite were replaced and coupled by HPHO after adsorption, which was in agreement with the FTIR results. The O 1s peaks around 533.1 and 531.3 eV further supported the conclusion that N—O—Cu and C—O—Cu bonds formed as already stated above.
Based on previous results and discussion, a schematic model of HPHO adsorption on a malachite surface is shown in Fig. 8. It is demonstrated that the Cu species are bound with O atoms in the —OH and C=N—OH groups, and d-pπ might be formed between Cu and C=C group. As a strong adsorption of HPHO though double conjugate structure assistance, the modified malachite is easily floated out in pulp.
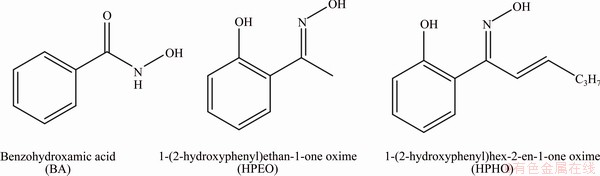
Fig. 7 Chemical structure of collectors used in this work
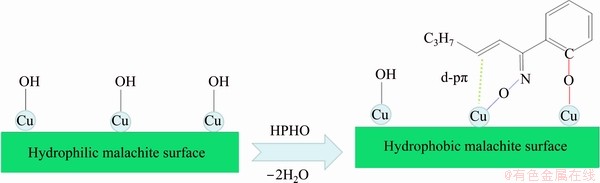
Fig. 8 Absorption model of HPHO on surface of malachite
5 Conclusions
(1) When the butenyl group was linked with 1-(2-hydroxyphenyl)ethan-1-one oxime, the collecting ability of HPHO was improved as we desired. The micro and batch flotation results indicated that HPHO was a special collector for malachite flotation. The micro-flotation results indicated that compared with SIBX, BA and HPEO, HPHO showed better flotation performance and floated out 98.1% malachite at a pH of 8.0. Batch flotation experiment indicated that HPHO upgraded 4.13% Cu with 85.15% recovery without desliming treatment. This indicated that HPHO had a huge potential to deal with low-grade copper ores in minerals engineering.
(2) The results of the zeta potential before and after HPHO attachment indicated a possible chemisorption. The FTIR and XPS analyses offered clear evidence that —OH groups on malachite surface were replaced and coupled by HPHO. And Cu(II)-HPHO complex was formed after HPHO adsorption. Detailed results of FTIR and XPS implied the C=C bond, O atoms in oxime group and benzene ring would fix with Cu species on malachite though d-pπ, C—O—Cu and N—O—Cu bonds.
References
[1] REGINA F G, MIOMIR G P, MILORAD V T. Effect of temperature on the corrosion inhibition of nonionic surfactant TRITON-X-405 on ferritic stainless steel in 1.0 M H2SO4 [J]. Industrial & Engineering Chemistry Research, 2012, 51: 274-284.
[2] GAO Li-li, YIN Hua-yi, ZHU Hua, MAO Xu-hui, GAN Fu-xing, WANG Di-hua. Separation of dispersed carbon nanotubes from water: Effect of pH and surfactants on the aggregation at oil/water interface [J]. Separation & Purification Technology, 2014, 129: 113-120.
[3] LI Shuang-ke, GU Guo-hua, QIU Guan-zhou, CHEN Zhi-xiang, Flotation and electrochemical behaviors of chalcopyrite and pyrite in the presence of N-propyl-N′- ethoxycarbonyl thiourea [J]. Transactions of Nonferrous Metals Society of China,2018, 28: 1241-1247.
[4] XIAO Wei, REN Ya-xin, YANG Juan, CAO Pan, WANG Jun, QIN Wen-qing, QIU Guan-zhou. Adsorption mechanism of sodium oleate and styryl phosphonic acid on rutile and amphibole surfaces [J]. Transactions of Nonferrous Metals Society of China, 2019, 29: 1939-1947.
[5] CORIN K C, KALICHINI M, O‘CONNOR C T, SIMUKANGA S. The recovery of oxide copper minerals from a complex copper ore by sulphidisation [J]. Minerals Engineering, 2017, 102: 15-17.
[6] HUANG Yao-guo, LIU Guang-yi, LIU Jun, YANG Xiang-lin, ZHANG Zhi-yong. Thiadiazole-thione surfactants: Preparation, flotation performance and adsorption mechanism to malachite [J]. Journal of Industrial and Engineering Chemistry, 2018, 67: 99-108.
[7] FENG Qi-cheng, ZHAO Wen-juan, WEN Shu-ming. Surface modification of malachite with ethanediamine and its effect on sulfidization flotation [J]. Applied Surface Science, 2018, 436: 823-831.
[8] LIU Guang-yi, HUANG Yao-guo, QU Xiao-yan, XIAO Jing-jing, YANG Xiang-lin, XU Zheng-he. Understanding the hydrophobic mechanism of 3-hexyl-4-amino-1, 2,4-triazole-5-thione to malachite by ToF-SIMS, XPS, FTIR, contact angle, zeta potential and micro-flotation [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2016, 503: 34-42.
[9] HOPE G A, BUCKLEY A N, PARKER G K, NUMPRASANTHAI R W, MCLEAN J. The interaction of n-octanohydroxamate with chrysocolla and oxide copper surfaces [J]. Minerals Engineering, 2012, 36-38(5): 2-11.
[10] YIN Wan-zhong, SUN Qian-yu, LI Dong, TANG Yuan, FU Ya-feng, YAO Jin. Mechanism and application on sulphidizing flotation of copper oxide with combined collectors [J]. Transactions of Nonferrous Metals Society of China, 2019, 29: 178-185.
[11] CECILE J L, CRUZ M I, BARBERY G, FRIPIAT J J. Infrared spectral study of species formed on malachite surface by adsorption from aqueous salicylaldoxime solution [J]. Journal of Colloid & Interface Science, 1981, 80: 589-597.
[12] XU Jin-qiu. A new collector for the flotation of wolframite [J]. Nonferrous Metals, 1989(2): 28-32. (in Chinese)
[13] ZHU Jian-guang, WU Xi-qing. Application of isomerization principle in synthetic oxidation oxide collectors [J]. Nonferrous Metals, 1990(3): 32-37. (in Chinese)
[14] CHENG K L, UENO K, IMSMURA T. Handbook of organic analytical reagents [M]. New York: CRC Press, 1982: 534.
[15] DAS B, RAVIPRASAD A. Flotation of Rakha (India) copper ore with LIX-65 NS [J]. Minerals and Metallurgical Processing, 1996, 13(2): 61-64.
[16] XU Jin-qiu, XU Xiao-jun. Synthesis of a new collector 2-hydroxy 1-naphthaldehyde oxime and its application in rare earth ore flotation [J]. Foreign Metal Ore Dressing, 2001, 38(5): 38-39. (in Chinese)
[17] XU Hai-feng, ZHONG Hong, WANG Shuai, NIU Ya-nan, LIU Guang-yi. Synthesis of 2-ethyl-2-hexenal oxime and its flotation performance for copper ore [J]. Minerals Engineering, 2014, 66-68: 173-180.
[18] LI Li-qing, ZHAO Jing-hua, XIAO Yan-yin, HUANG Zhi-qiang, GUO Zhi-zhong, LI Fang-xu, DENG Lan-qing. Flotation performance and adsorption mechanism of malachite with tert-butylsalicylaldoxime [J]. Separation and Purification Technology, 2019, 210: 843-849.
[19] SAIKIA L, BARUAH J M, THAKUR A J. A rapid, convenient, solventless green approach for the synthesis of oximes using grindstone chemistry [J]. Organic and Medicinal Chemistry Letters, 2011, 1(1): 12.
[20] ZDENEK T, EVA V, JAROSLAV K, DAVID K. Aldol condensation of benzaldehyde and heptanal over zinc modified mixed Mg/Al oxides [J]. Catalysis Letters, 2018, 148: 2042-2057.
[21] HYODO K, TOGASHI K, OISHI N, GENNA H, KINGO U. Bronsted acid catalyzed transoximation reaction: Synthesis of aldoximes and ketoximes without use of hydroxylamine salts [J]. Green Chemistry, 2016, 18: 5788-5793.
[22] LI Fang-xu, ZHONG Hong, XU Hai-feng, JIA Hui, LIU Guang-yi. Flotation behavior and adsorption mechanism of α-hydroxyoctyl phosphinic acid to malachite [J]. Minerals Engineering, 2015, 71: 188-193.
[23] ALEXANDRATOS S D, ZHU X, FLORENT M, SELLIN R. Polymer-supported bifunctional amidoximes for the sorption of uranium from seawater [J]. Industrial & Engineering Chemistry Research, 2016, 55: 4208-4216.
[24] SAHA B, DAS G. Malachite nanoparticle: A new basic hydrophilic surface for pH-controlled adsorption of bovine serum albumin with a high loading capacity [J]. Journal of Physical Chemistry C, 2009, 113: 15667-15675.
[25] RAMESH V, JAN B, PAULV J, STEPHEN Z, NEIL B. Relationship between fundamental interfacial properties and foaming in linear and branched sulfate, ethoxysulfate, and ethoxylate surfactants [J]. Journal of Colloid & Interface Science, 1990, 140(1): 31-34.
[26] WANG Zhou-jie, WU Hou-qin, XU Yan-bo, SHU Kai-qian, JIE Yang, LUO Li-ping, XU Longhua. Effect of dissolved fluorite and barite species on the flotation and adsorption behavior of bastnaesite [J]. Separation and Purification Technology, 2020,237: 116387.
[27] TIAN Jia, XU Long-hua, SUN Wei, ZENG Xiao-bo, FANG Shuai, HAN Hai-sheng, HONG Kai, HU Yue-hua. Use of Al2(SO4)3 and acidified water glass as mixture depressants in flotation separation of fluorite from calcite and celestite [J]. Minerals Engineering, 2019, 137: 160-170.
[28] WANG Zhou-jie, WU Hou-qin, XU Yan-bo, SHU Kai-qian, FANG Shuai, XU Long-hua. The effect of dissolved calcite species on the flotation of bastnaesite using sodium oleate [J]. Minerals Engineering, 2020, 145: 106095.
李方旭1,2,3,4,周晓彤1,2,3,4,林日孝1,2,3,4
1. 广东省科学院,广州 510650;
2. 广东省资源综合利用研究所,广州 510650;
3. 稀有金属分离与综合利用国家重点实验室,广州 510650;
4. 广东省矿产资源开发和综合利用重点实验室,广州 510650
摘 要:以2-羟基苯乙酮和丁醛为原料合成一种新型1-(2-羟苯基)己-2-烯-1-酮肟(HPHO)捕收剂。通过浮选试验、zeta电位、傅里叶变换红外光谱(FTIR)和X射线光电子能谱(XPS)分析技术研究HPHO对孔雀石的浮选性能和吸附机理。结果表明,与苯甲羟肟酸(BA)、1-(2-羟苯基)乙-1-酮肟(HPEO)和异丁基黄药(SIBX)相比,HPHO对孔雀石具有更好的捕收性能且无需额外添加调整剂Na2S和起泡剂甲基异丁基甲醇(MIBC)。Zeta电位结果发 现,HPHO可能通过化学作用吸附在孔雀石表面。 FTIR和XPS结果表明,HPHO通过C=C、—OH和N—OH基团与孔雀石表面的铜物种键合形成Cu-肟配合物。
关键词:孔雀石;浮选;捕收剂;肟;吸附机理
(Edited by Wei-ping CHEN)
Foundation item: Projects (2018GDASCX-0934, 2020GDASYL-20200302009) supported by Guangdong Academy of Sciences, China
Corresponding author: Xiao-tong ZHOU; Tel: +86-13826281796; E-mail:zxtonghao@126.com
DOI: 10.1016/S1003-6326(20)65421-8

