
Characteristics and electrochemical performance
of cathode material Co-coated LiNiO2 for Li-ion batteries
ZHONG Sheng-wen(钟盛文)1, 2, ZHAO Yu-juan(赵煜娟)1, LIAN Fang(连 芳)1, LI Yan(李 艳)1,
HU Yang(胡 杨)1, LI Pei-zhi(李培植)2, MEI Jia(梅 佳)2, LIU Qing-guo(刘庆国)1
1. Laboratory on Solid State Ionics, University of Science and Technology Beijing, Beijing 100083, China;
2. Faculty of Materials and Technology Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, China
Received at 28 February 2005; accepted 20 December 2005
Abstract: Spherical Ni(OH)2 powder coated with Co(OH)2 as raw material was mixed with LiOH to synthesize cathode material for lithium ion battery by using solid-state reaction. After sintered at temperature above 600 ℃, a solid solution with layer structure was formed. The result of XPS shows that it is a concentration gradient material with higher cobalt content at the surface, and the gradient decreases with increasing sintering temperature from 650 to 750 ℃. This new gradient material, called as Co-coated LiNiO2, exhibits excellent electrochemical performances for the cathode of Li-ion batteries in comparison with LiNiO2 and Co-doping LiNiO2. The discharge capacity of Co-coated LiNiO2 is over 180 mA·h/g and capacity decay per cycle is less than 0.07% when Co-coated LiNiO2 consisting of 92% nickel and 8% cobalt was sintered at the temperatures between 650-670 ℃. Though initial discharge capacity could be increased with higher sintering temperature, the cycle life would be reduced. Key words: Li-ion battery; LiNiO2; LiCoO2; Ni(OH)2; Co(OH)2; cathode material; gradient cathode material; electrochemical performance
1 Introduction
LiNiO2 was considered as a promising cathode material for Li-ion batteries due to its high discharge capacity and low cost in comparison with LiCoO2. However, the structural change occurred during charging and discharging would deteriorate the electrochemical characteristics and resulted in high capacity decay[1]. In the fully charging state, LixNiO2 with low lithium content (x<0.3) is unstable and might generate oxygen, which would trigger the exothermic oxidation reaction of electrolyte, resulting in safety problem and particle fracture[2, 3].

 To improve the cycle performance and safety of LiNiO2, Co-doping[4-8], Al-doping[9, 10], multiple- ion doping[11, 12], surface treatment[13], and other methods [14-18] had been adopted. Although the cycle performance of LiNiO2-based cathode could be improved by the doping technique, the performance is not satisfactory according to the capacity, cycle life and stability. In this paper the electrochemical performance of LiNiO2-based cathode was tried to be improved by coating, rather than the uniform doping. As the surface would dominate the characteristics of cathode particles, surface coating may show much efficient effect.
To improve the cycle performance and safety of LiNiO2, Co-doping[4-8], Al-doping[9, 10], multiple- ion doping[11, 12], surface treatment[13], and other methods [14-18] had been adopted. Although the cycle performance of LiNiO2-based cathode could be improved by the doping technique, the performance is not satisfactory according to the capacity, cycle life and stability. In this paper the electrochemical performance of LiNiO2-based cathode was tried to be improved by coating, rather than the uniform doping. As the surface would dominate the characteristics of cathode particles, surface coating may show much efficient effect.
A series of Co-coated LiNiO2 was synthesized in this work. The test result indicates that it is a concentration gradient material with higher cobalt content at the surface. This new gradient cathode exhibits excellent electrochemical performance in com- parison with LiNiO2 and doping LiNiO2.
2 Experimental
The Ni(OH)2 powder coated with Co(OH)2 was prepared with spherical Ni(OH)2 powder (supplied from Kelong Power Sources Co., Xinxiang of Henan, China) via controlled-precipitation technique. Spherical Ni(OH)2 particles were suspended in a water bath containing ammonia at 50 ℃, then an appropriate amount of CoSO4 solution was added into the suspension. By means of slowly adding NaOH solution, controlling the PH value, temperature and adequate stir, a Co(OH)2 layer could be precipitated on the surface of spherical Ni(OH)2 particles. After washing, centrifugal filtering and drying at 95 ℃, the Co(OH)2-coating Ni(OH)2 powder was obtained with mole ratio of Co to (Co+Ni) at 0.05, 0.10, 0.15.
Such produced Co(OH)2-coating Ni(OH)2 powder has the core-shell structure to be used as precursors to synthesize Co-coated LiNiO2 by solid-state technique. This precursor and LiOH were weighed and mixed with 4% excess of lithium. The mixture was fired at 600 ℃ for 20 h. After cooling with furnace, the fired mixture was crashed by a centrifugal crasher to get fine powder with size of less than 60 μm. The fine powder was sintered again at different temperatures of 600, 650, 700 and 750 ℃ for 30 h in oxygen atmosphere, then slowly cooled to 480 ℃ for 10 h, and then cooled with furnace. The sintered product was crashed again to particle with size of less than 60 μm. It is found that such produced powder is a concentration gradient material with high cobalt concentration at the particle surface. So, it is called as Co-coated LiNiO2. An airflow classifier was used to remove extra- fine and too large particles. The produced Co-coated LiNiO2 had the particle size between 2-20 μm.
Such produced resultants of Co-coated LiNiO2 were mixed with 4%(mass fraction, the same below if not mentioned) carbon black and 6% polyvinylidene fluoride (PVDF) (emulsion in N-methylic pyrrolidone) in a stirring machine to get cathode paste. The paste was coated on an aluminum foil by a blade coater. After press-rolling and drying at 140 ℃ for 8 h, the cathode film was ready to be used to assemble lithium ion batteries. The anode material MCMB (meso-carbon micro-beat, supplied by Shanshan Science and Technology Co Ltd, Shanghai, China) mixed with 4% carbon black and 6% PVDF was coated on a copper foil as the anode film. 1 mol/L LiPF6/EC+DMC (1∶1 in volume, from Zhangjiagang Xiangda Battery Materials Co Ltd, China) was used as the electrolyte, and Celgard 2300 as separator. AA size MCMB/ Co-coated LiNiO2 batteries were assembled in dry air box (dew point< -40 ℃). The cycling was processed with CC-CV model (constant current charging to upper voltage (4.20 V), then constant voltage charging at the upper voltage) between 2.75 and 4.20 V with 1C rate.
The Co-coated LiNiO2 powder was characterized by X-ray (Rigaku D/max-2400) with Cu Kα radiation. The XRD data was collected at the step of 0.02° in the diffraction ?range from 10° to 90°. Particle sizes, shapes and morphology were observed by scanning electron microscopy (SEM, S-360) and transmission electron microscopy (TEM, 100CX). The cobalt content in the coated-LiNiO2 particles was analyzed by energy dispersive X-ray detection (EDX).
3 Results and discussion
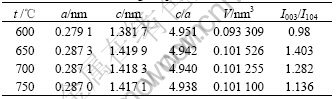
It can be seen from Fig.1 that the produced Co- coated LiNiO2 particles possess the excellent spherical shape. The average particle size of produced resultant is about 10 μm analyzed by a laser particle distribution analyzer. The XRD patterns of different Co-coated LiNiO2 powders sintered at temperature from 600 to 750 ℃ are shown in Fig.2. It can be seen that the Co-coated LiNiO2 shows α-NaFeO2 structure (R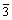 m), the same as LiNiO2 and LiCoO2. From Fig.2, it seems that only one phase appears and a solid solution forms after sintering. When sintered at 600 ℃, the main spectrum peaks, such as I003 and I104, are lower and wider, which indicates that crystallization of Co-coated LiNiO2 particles is not perfect. When samples are sintered at temperatures above 650 ℃, the diffraction spectra are sharp. Table 1 lists the lattice parameters calculated from XRD. It can be seen that the lattice parameters a and c, the ratio of c/a, as well as I003/I104 and V (cell volume) becomes smaller during sintering temperature from 650 to 750 ℃.
m), the same as LiNiO2 and LiCoO2. From Fig.2, it seems that only one phase appears and a solid solution forms after sintering. When sintered at 600 ℃, the main spectrum peaks, such as I003 and I104, are lower and wider, which indicates that crystallization of Co-coated LiNiO2 particles is not perfect. When samples are sintered at temperatures above 650 ℃, the diffraction spectra are sharp. Table 1 lists the lattice parameters calculated from XRD. It can be seen that the lattice parameters a and c, the ratio of c/a, as well as I003/I104 and V (cell volume) becomes smaller during sintering temperature from 650 to 750 ℃.

Fig.1 SEM image of Co-coated LiNiO2 powders sintered at 650 ℃ for 12 h (Molar ratio of Co to Co and Ni is 0.10)
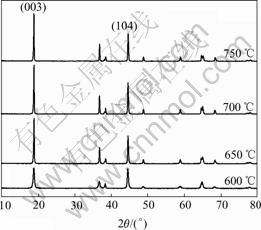
Fig.2 XRD patterns of Co-coated LiNiO2 sintered at different temperatures
Table 1 Crystal lattice parameters for synthesized Co-coated LiNiO2 at different sintering temperatures
To know the distribution of cobalt element in the spherical particle of Co-coated LiNiO2 sintered at different sintering temperatures, the micro-concentration of cobalt is analyzed by EDX. Fig.3 shows the Co concentrations in the spherical particles from the surface to center. It is verified that during the sintering process the cobalt atoms diffuse from surface into the matrix, and a solid solution forms. The cobalt concentration at the surface decreases with the increasing of the sintering temperature. When sintered at 600 ℃ for 20 h, there is a much higher cobalt concentration (about 0.15) near the particle surface than that in the particle center (about 0.02). When the sintering temperature reaches 650 ℃, a high cobalt concentration near the particle surface still maintains, but the cobalt concentration at the center and bulk of the particle increases. However, it is clear that it remains the characteristics of a concentration gradient material when sintered at 650 ℃. When the sintering temperature is above 700 ℃, the concentration difference of cobalt from surface to center does not become remarkable due to the large diffusion rate. When the sintering temperature is above 750 ℃ for 20 h, the cobalt concentration becomes uniform through the total particle. It is important that the sintering temperature and duration time should be precisely controlled to achieve the characteristics of the concentration gradient material. The sintering temperature could not be too high to ensure the surface having higher concentration of cobalt, moreover, the sintering temperature should be high enough to complete the synthetic reaction, resulting in good crystallization. According to our experimental results, the optimum sintering temperature is 650-670 ℃ for 20 h.
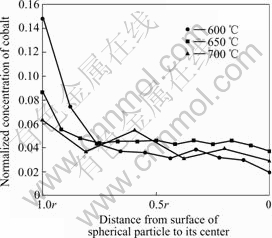
Fig.3 Distribution of Co element in spherical coated-LiNiO2 particle at different sintering temperatures
Fig.4 shows the cycle performances of Co-coated LiNiO2 sintered at different temperatures. The cycle performance of cathode samples sintered at 600 ℃ and 750 ℃ for 20 h are inferior, further showing low capacity and laterly having very high capacity decay. The synthetic reaction for the sample sintered at 600 ℃ might not be completed. When sintered at 750 ℃, cobalt diffuses to the center, losing the concentration gradient, and more divalent nickel NI2+ would be generated at high temperature, which might occupy the location of lithium and deteriorate the electrochemical performances. The cycle performances of the samples sintered at 650 and 700 ℃ are excellent, further shows good cycle life, no capacity decay for 20 cycles, laterly provides higher capacity.
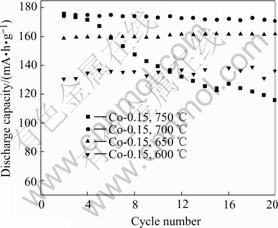
Fig.4 Cycle performances of Co-coated LiNiO2 cathode materials sintered at different temperatures
The test results with AA size lithium batteries are shown in Figs.5 and 6. It can be seen that the Co-coated enables the LiNiO2 cathode to be greatly improved. The discharge capacity of 190 mA·h/g and good cycle life confirms that the particle surface shows dominative effect on the electrochemical performances. In our previous works, Co and Al-doped LiNiO2 (uniform distribution of Co and Al in LiNiO2) shows good improvement on the cycle life, but the reversible capacity decreases.
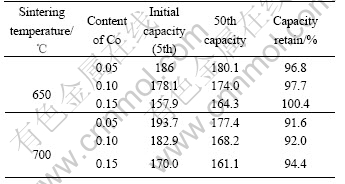
For a clear comparison, the discharge capacity of Co-coated LiNiO2 cathode materials sintered at 650 and 700 ℃ with Co contents of 0.05, 0.10 and 0.15 are summarized in Table 2. It is clear that with the increasing sintering temperatures from 650 to 700 ℃, the discharge capacity increases, and with increasing molar ratio of Co to Co and Ni from 0.05, 0.10 to 0.15, the reversible capacity decreases, but showing better cycle life. The cathode material sintered at 650 ℃ shows excellent discharge capacity and good cycle life. When the sintering temperature increases to 700 ℃, there is a higher discharge capacity, but a little worse cycling performance. When the sintering temperature increases to 750 ℃ its initial discharge capacity still remains at high value, but its capacity drops sharply.
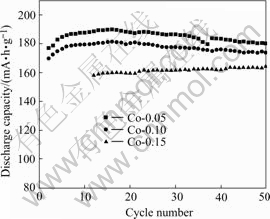
Fig.5 Cycling performance of Co-coated LiNiO2 sintered at 650 ℃
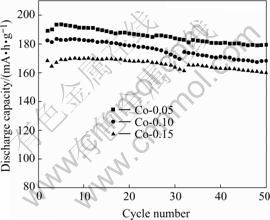
Fig.6 Cycling performance of Co-coated LiNiO2 sintered at 700 ℃
Table 2 Cycling performances of Co-coated LiNiO2 cathode materials (mA·h·g-1)
To pursue excellent electrochemical performances the synthesis reaction should be processed at a high temperature to ensure the formation of the perfect layer structure, but not too high, resulting in the absence of cobalt concentration gradient, as the high cobalt concentration at surface might suppress the formation of Ni2+.
4 Conclusions
1) A new cathode material for lithium ion batteries was developed. It is a Co-coated LiNiO2 gradient material with high cobalt concentration at the surface. This new cathode material shows high capacity above 180 mA·h/g and excellent cycle performances.
2) The new gradient cathode material was synthesized with LiOH and spherical nickel hydroxide powder embedded in cobalt hydroxide. The synthesis temperature and duration time are two important parameters. To ensure the formation of a perfect layer structure, the sintering temperature should be high enough, but not too high, resulting in the absence of cobalt concentration gradient. The high cobalt concentration at surface might suppress the formation of Ni2+ and derangement of Li+ and Ni2+, which would deteriorate the electrochemical performances of LiNiO2-based cathode materials.
Acknowledgement
We are glad to express our appreciation to the colleagues in Shijiazhuang Best Battery Materials Co Ltd for their help and support of the present work.
References
[1] Hirano A, Kanho R, Kawamoto Y, et al. Relationship between non-stoichiometry and physical properties in LiNiO2 [J]. Solid State Ionics, 1995, 78: 123-131.
[2] Kaoru D, Matsuhiko N, Soichin H, et al. In situ observation of LiNiO2 single-particle fracture during Li-ion extraction and insertion [J]. Electrochemical and Solid-state Letters, 2000, 3: 125-127.
[3] TIAN Yan-wen, LI Hui, ZHANG Xin, ZHAI Yu-chun, GAO Hong. Synthesis and thermal decomposition kinetics of LiNiO2 [J]. Trans Nonferrous Met Soc China, 2002, 12(1): 127-131.
[4] Delmas C, Saadoune I. Electrochemical and physical properties of the LiNi1-yCoyO2 phases [J]. Solid State Ionics, 1992, 53-56: 370-375.
[5] Fujita Y, Amine K. LiNi1-yCoyO2 prepared at low temperature using and either LiNO3 or LiOH [J]. J Power Sources, 1997, 68: 126-130.
[6] Chang Chun-Chieh, Scarr N, Kumta P N. Synthesis and electrochemical characterization of LiMO2 (M=Ni,Ni0.75Co0.25) for rechargeable lithium ion batteries [J]. Solid State Ionics, 1998, 112: 329-344.
[7] Wang G X, Horvat J, Bradhurst D H, Liu H K, Dou S X. Structure, physical and electrochemical characterization of LiNixCo1-xO2 solid solutions [J]. J Power Sources, 2000, 85: 279-283.
[8] Kinoshita A, Yanagida K, Yanai A, et al. Electrochemical characteristics of LiNi1-xCoxO2 as positive electrode material for lithium secondary batteries [J]. J Powder Sources, 2001, 102: 283-287.
[9] Amriou T, Seyede A, Khelifa B, et al. Effect of Al-doping on lithium nickel oxides [J]. J Powder Sources, 2004, 130: 213-220.
[10] Chen C H, Liu J, Stoll M E, Henriksen G, Vissers D R, Amine K. Aluminum-doped lithium nickel cobalt oxide electrodes for high-power lithium-ion batteries [J]. J Powder Sources, 2004, 128: 278-285.
[11] Kannan A M, Manthiran A. Structural stability of Li1-xNi0.85Co0.15O2 and Li1-xNi0.85Co0.12Al0.03O2 cathodes at elevated temperature [J]. J of the Electrochemical Society, 2003, 150: A349-A353.
[12] Wang G X, Bewlay S, Yao J, et al. Multiple-ion-doped lithium nickel oxides as cathode materials for lithium-ion batteries [J]. J Powder Sources, 2003, 119-121: 189-194.
[13] Ying J R, Wan C R, Jiang C Y. Surface treatment of LiNi0.8Co0.2O2 cathode materials for lithium secondary battery [J]. J Powder Sources, 2001, 102: 162-166.
[14] Julien C, Massot M. Raman scattering of LiNi1-yAlyO2 [J]. Solid State Ionics, 2002, 148: 53-59.
[15] Stoyanova R, Zhecheva E, Alcantara R, et al. Lithium/nickel mixing in the transition metal layers of lithium nickelate: high-pressure synthesis of layered Li[LixNi1-x]O2 oxides as cathode materials for lithium-ion batteries [J]. Solid State Ionics, 2003, 161: 197-204.
[16] Smart M C, Ratnkumar B V, Whitcanack L D, et al. Improved low-temperature performance of lithium-ion cells with quaternary carbonate-based electrolyte [J]. J Powder Sources, 2003, 119-121: 349-358.
[17] Yoshinori K, Akria K, Katsunri Y. Study on capacity fade factors of lithium secondary batteries using Li1Ni0.7Co0.3O2 and graphite-coke hybrid carbon [J]. Electrochimica Acta, 2002, 47: 4157-4162.
[18] LI Hui, ZHAI Yu-chun, TIAN Yan-wen. Preparation of LiNi1-yCoyO2 in certain oxygen pressure [J]. Trans Nonferrous Met Soc China, 2003, 13(5): 1040-1045.
Corresponding author: LIU Qing-guo; Tel/Fax: +86-10-62334861; E-mail: zhongshw@126.com
(Edited by LONG Huai-zhong)