文章编号:1004-0609(2009)09-1695-05
含氯低pH值溶液中BDT自组装膜对铜电极的缓蚀行为
黄文章1,童耀斌2,罗红群2,李念兵2,李 丹1
(1. 重庆科技学院 化学化工学院,重庆 401331;
2. 西南大学 化学化工学院,重庆 400715)
摘 要:采用分子自组装技术,在铜电极表面形成一层1, 3-二巯基硫醇(BDT)单分子自组装膜。通过交流阻抗、极化曲线和循环伏安等电化学方法探讨该自组装膜在3%NaCl溶液中对铜电极的缓蚀作用。实验表明:BDT能够有效地组装到铜的表面形成单分子自组装膜,BDT自组装膜能有效地抑制铜基底在3%NaCl腐蚀介质以及酸性NaCl溶液中的腐蚀行为;缓蚀效率随组装时间的延长逐渐升高并趋于稳定,在pH值较低的溶液中仍具有较好的缓蚀效果;当BDT浓度为1.0×10-2 mol/L、组装时间为40 h时,铜电极的腐蚀电流最小,缓蚀效率最好。
关键词:1, 3-二巯基硫醇;自组装膜;铜电极;缓蚀
中图分类号:TG 174.42 文献标识码:A
Corrosion inhibition behavior of copper electrode by self-assembled monolayer of BDT in solution with chlorine and low pH value
HUANG Wen-zhang1, TONG Yao-bin2, LUO Hong-qun2, LI Nian-bing2, LI Dan1
(1. School of Chemistry and Chemical Engineering, Chongqing University of Science and Technology,
Chongqing 401331, China;
2. School of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715, China)
Abstract: Adopting molecular self-assembly technique, a self-assembled membrane of 1, 3-benzenedithiol (BDT) was formed on the surface of the copper electrode. The effect of this membrane on the corrosion inhibition of copper electrode in 3% NaCl solution was investigated by using electrochemical impedance spectroscopy (EIS), polarization curve and cyclic voltammetry (CV). The results show that BDT can be assembled on the surface of copper electrode to form monomolecular self-assembled membrane,and the membrane can efficiently inhibit the copper base corrosion in the 3% NaCl corrosion medium and acidic solution. The inhibition efficiency increases with increasing assembling time and obtains steady state gradually. In the solution with lower pH value, BDT can also keep good inhibitor. When the copper electrode is modified with 1.0×10-2 mol/L BDT for 40 h, the corrosion current is the smallest, the inhibition efficiency is the highest.
Key words: 1, 3-benzenedithiol; self-assembled monolayer; copper electrode; corrosion
金属铜具有良好的导热、导电性和机械加工性能,因此,铜广泛应用于工业中。在通常情况下,铜的耐蚀性较好,但在含氯离子的溶液中会被严重腐蚀。氯离子比某些钝化剂更容易吸附在金属表面或在薄的钝化膜上形成强电场,使金属离子易于溶出或者与金属生成氯的络合物,加速金属溶解[1-2]。
目前,应用于腐蚀的有机物主要是含有未配对的氮﹑硫和氧原子的立体环状有机物,其中苯并三氮唑(BTA)应用比较广泛[3-7]。但BTA对铜起缓蚀作用的最佳pH值为6~11,但在pH值较低的一些介质中,BTA的缓蚀效率急剧下降,使其应用范围受到限制。
1992年,SABATANI和RUBINSTEIN[8]以及BLJIEV和HUBIN[9]报道了在空气中单层膜覆盖的Cu的腐蚀电阻,从此自组装开始用于防腐蚀研究。自组装膜是一类高度有序的分子组装体系,拥有良好的稳定性和有序性在金属腐蚀防护方面有广阔的应用前 景[10-12]。因硫醇自组装膜具有制备简单、有序性高等特点而成为自组装体系中的研究热点,且已取得较大进展[13-15]。硫醇类化合物能通过较强的化学键与铜表面结合,生成致密的膜,从而有效地抑制膜层下铜的腐蚀。本文作者通过交流阻抗、极化曲线和循环伏安法等电化学方法研究了BDT的浓度和组装时间对含氯离子和低pH值含氯离子溶液中BDT自组装膜对铜的缓蚀作用。
1 实验
1.1 实验材料
BDT购于Sigma公司;氯化钠(NaCl)购于重庆北碚化学试剂厂。用无水乙醇溶解配成2 mmol/L的 BDT溶液;用去离子水配制3%的NaCl溶液。所用试剂均为分析纯。
1.2 自组装膜的制备
先将铜电极用0.3和 0.05 mm Al2O3粉末打磨、抛光,之后依次用超纯水、乙醇超声清洗干净,然后置入上述BDT溶液中进行组装。组装之后将电极干燥 1 h,最后放入3%的NaCl溶液中,待电位稳定后进行电化学性能测试。
1.3 测试方法
所有电化学测试和数据分析,由AUTOLAB (PGSTAT302)电化学工作站(荷兰)完成。采用三电极体系:选用d=2 mm的铜电极(纯度99.99%,天津艾达科技发展有限公司)为工作电极,参比电极为Ag/AgCl,铂丝电极为辅助电极。电化学阻抗谱测量频率范围为20 Hz~60 kHz。循环伏安法的扫描速度为20 mV/s,选择-0.5 V作为初始扫描电位,从负向正扫描至最高电位0.6 V,然后反向扫描至负的最大值-0.6 V,再正向扫描返回初始电位。极化曲线的扫描速度为1 mV/s,扫描的电位范围为-0.4~0.1 V,实验在室温下进行。
2 结果与讨论
2.1 交流阻抗测试
图1所示为裸铜(Cu)电极在3%NaCl溶液中的阻抗谱。由图1可知,在Nyquist曲线的高频区,有一个Rt很小的半圆,表明空白电极上电化学反应的速度很快;低频区出现Warburg阻抗,表明腐蚀过程中存在着扩散传质过程。

图1 pH=7时裸铜电极在3%NaCl溶液中的阻抗曲线
Fig.1 Impedance curve of bare Cu electrode in 3%NaCl at pH value of 7
图2所示为自组装BDT膜的铜电极(BDT/Cu)在3%的NaCl溶液中的阻抗曲线。由图2可知,自组装后的铜电极阻抗达1 M?,且未出现Warburg阻抗,与空白电极相比,阻抗显著增加,表明铜基表面的自组装BDT膜致密,对NaCl溶液的浸蚀具有有效抵御作用。

图2 pH=7时BDT/Cu电极在3%NaCl溶液中的阻抗曲线
Fig.2 Impedance curve of BDT/Cu electrode in 3%NaCl at pH value of 7
由于电荷转移电阻Rt与有机膜在铜表面的覆盖度θ有关,θ和评价缓蚀效率的缓蚀率η可由下式得到:


式中: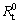 为裸电极电荷传递电阻;Rt为具有自组装膜的电极电荷传递电阻;v0为不含缓蚀剂时铜的腐蚀速率;v为含有缓蚀剂时铜的腐蚀速率。
为裸电极电荷传递电阻;Rt为具有自组装膜的电极电荷传递电阻;v0为不含缓蚀剂时铜的腐蚀速率;v为含有缓蚀剂时铜的腐蚀速率。
由裸电极和组装BDT膜的铜电极的阻抗曲线以及由式(1)和(2)计算得到的阻抗值及缓蚀效率列于表1。
表1 裸铜电极和BDT/Cu电极在3%NaCl溶液中的阻抗曲线拟合与计算结果
Table 1 Fitting results of impedance curves and calculation results of bare Cu and BDT/Cu electrode

由表1可知,组装BDT后的铜电极表面BDT的覆盖度为99.88%,表明自组装BDT可有效降低铜电极在3%NaCl溶液中的腐蚀速率。
2.2 电化学极化曲线测试
图3所示为BDT/Cu和Cu电极在3%NaCl溶液中的电化学极化曲线对比。图中曲线a为裸Cu电极的极化曲线,曲线b为组装BDT后Cu电极的电极化曲线。从图3可知,组装BDT后,Cu电极的腐蚀电流显著下降,阳极极化和阴极极化塔菲尔曲线的斜率明显增大。

图3 pH=7时Cu电极和BDT/Cu电极在3%NaCl溶液中的极化曲线
Fig.3 Polarization curves of bare Cu and BDT/Cu electrode in 3% NaCl solution at pH value of 7
对图3中曲线中的腐蚀电流进行拟合,用式(3)计算可得到自组装的缓蚀效率η

图3的电极极化曲线拟合与计算结果列于表2。由表2中的缓蚀效率可以看出,BDT在铜表面形成了致密的自组装膜,实验进一步印证了前面交流阻抗的结果。
表2 电极极化曲线拟合与计算结果
Table 2 Fitting results of polarization curves and calculation results of electrode

2.3 循环伏安法测试
图4所示为Cu电极以及BDT/Cu电极在NaCl溶液中循环伏安曲线。Cu在NaCl溶液中氧化还原过程中发生的反应如下:

其中,在正向扫描过程中主要发生铜的溶解、络合和成膜,即反应(4)~(6)。反应式中CuCla为Cu的吸附络合;CuClf为Cu的成膜。反应(7)~(9)代表负向扫描过程中发生表面腐蚀产物的消长过程。

图4 pH=7时Cu电极和BDT/Cu电极在3%NaCl溶液中的循环伏安曲线
Fig.4 CV curves of bare Cu and BDT/Cu electrode in 3%NaCl solution at pH value of 7
Cu电极在正向扫描曲线上分别在0.03和0.267 V得到两个氧化电流峰A1和B1,在反向扫描曲线上于-0.251 V的电位处得到一个大的还原电流峰C1。氧化峰A1与反应式(4)和(5)有关,表示CuCl盐膜层的形成;氧化峰B1与反应式(6)有关,归因于Cu+的进一步氧化和Cu2+的氧化物(或者氢氧化物)在电极表面的覆盖,还原峰C1的形成则与反应式(7)~(9)有关,对应于铜腐蚀产物的还原。
当铜电极的表面被BDT自组装膜覆盖后,电极的伏安行为发生很大的变化。自组装膜覆盖的铜基底的电化学氧化反应只能发生在自组装膜中的缺陷部位,与Cu电极相比,氧化-还原过程所对应的电流明显下降。
2.4 组装时间对交流阻抗的影响
固定BDT的浓度为1.0×10-2 mol/L,考察了BDT在Cu电极表面不同组装时间对3%NaCl腐蚀介质中交流阻抗的影响,其结果如图5所示。

图5 pH=7时在Cu电极表面不同自组装时间对3%NaCl腐蚀介质中交流阻抗值的影响
Fig.5 Effect of self-assembled time on impedance in 3%NaCl solution at pH=7 on surface of Cu electrode
由图5可知,在最初的组装时间内Rt增长很快,40 h后达到一个恒定值。这表明BDT在Cu表面的自组装过程是一个缓慢的过程,经过40 h的组装后形成了稳定致密的自组装膜,因此,实验选取组装时间为40 h。
2.5 酸性介质对缓蚀效率的影响
实验对BDT自组装铜电极在pH值较低的3%NaCl溶液中的腐蚀行为进行了研究。图6所示为BDT自组装铜电极在不同pH值下NaCl溶液中的Nyquist曲线。由图6可知,对比图1,当溶液的pH值逐渐降低时,曲线a、b和c上的半圆直径逐渐减小,说明Rt逐渐减小,缓蚀效果逐渐减弱,但没有Warburg阻抗出现且Rt较裸铜电极高,这说明没有扩散传质过程发生,膜还具有良好的抗腐蚀能力。
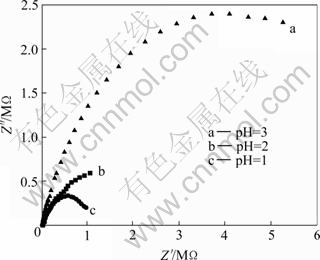
图6 不同pH值时BDT/Cu电极在3%NaCl溶液中的阻抗曲线
Fig.6 Impedance curves of BDT/Cu electrode in 3%NaCl solution at different pH values
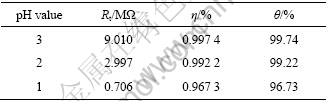
图6中极化曲线的分析结果列于表3。由表3可知,铜自组装BDT后在酸性NaCl溶液中,缓蚀效率和覆盖度虽有轻微下降,但仍在96%以上,表明自组装BDT的铜电极在酸性NaCl溶液中抗电化学腐蚀性较好。
表3 不同pH值时BDT/Cu电极在3%NaCl溶液中阻抗曲线的拟合与计算结果
Table 3 Fitting results of impedance curves and calculation results of BDT/Cu electrode in 3%NaCl solution at different pH values
3 结论
1) 采用分子自组装技术,可在Cu基表面组装 1, 3-二巯基硫醇自组装单分子膜。
2) BDT自组装膜能有效地抑制铜基底在3%NaCl腐蚀介质中的腐蚀行为。
3) BDT自组装膜在酸性NaCl溶液中,具有较好的抗腐蚀性能,在pH值较低的酸性条件下仍对铜起到良好的缓蚀作用。
REFERENCES
[1] BASTIDAS J M, PINILLA P. Copper corrosion inhibition by triphenylmethane derivatives in sulphuric acid media[J]. Corrosion Science, 2003, 45(2): 427-49.
[2] WANG C T, CHEN S H. Protection of copper corrosion by carbazole and N-vinylcarbazole self-assembled films in NaC1 solution[J]. Journal of Applied Electrochemistry, 2003, 33(2): 179-186.
[3] QUAN Z L, CHEN S H, ZHAO S Y, LI S L. Protection of copper corrosion by modification of self-assembled films of Schiff bases with alkanethiol[J]. Corrosion Science, 2001, 43(6): 1071-1080.
[4] 张胜涛, 薛茗月, 王艳波, 侯保荣. 苯并三氮唑缓蚀剂在铜表面覆盖行为的研究[J]. 腐蚀科学与防护技术, 2006, 18(5): 313-316.
ZHANG Sheng-tao, XUE Ming-yue, WANG Yan-bo, HOU Bao-rong. Study on covering behavior of BTA inhibitor on pure copper surface[J]. Corrosion Science and Protection Technology, 2006, 18(5): 313-316.
[5] 鞠 虹, 李 焰. 杂环化合物对热浸镀锌铝合金镀层在稀盐酸中的缓蚀作用[J]. 中国有色金属学报, 2008, 18(1): 177-186.
JU Hong, LI Yan. Corrosion inhibition of hot dipping Zn-Al alloy coated steels by heterocyclic compounds in diluted HCl solutions[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(1): 177-186.
[6] 郑红艾, 张大全, 邢 婕. HCl 溶液中氨基酸类化合物对铜的缓蚀作用[J]. 腐蚀与防腐, 2007, 28(12): 607-609.
ZHENG Hong-ai, ZHANG Da-quan, XING Jie. Effect of amino acid compounds in inhibitor on copper in HCl solution[J]. Corrosion & Protection, 2007, 28(12): 607-609.
[7] 杨生荣, 任嗣利, 张俊彦, 张绪寿. 自组装单分子膜的结构及其自组装机理[J]. 高等学校化学学报, 2001, 22(3): 470-476.
YANG Sheng-rong, REN Si-li, ZHANG Jun-yan, ZHANG Xu-shou. Structure and self-assembly mechanism of self-assembled monolayers[J]. Chemical Research in Chinese Universities, 2001, 22(3): 470-476.
[8] SABATANI E, RUBINSTEIN I. Electrochemical impedance analysis of polyaniline films on electrodes[J]. Journal of the Electrochemical Society, 1987, 134(12): 3078-3083.
[9] BLJIEV O, HUBIN A. Inhibition of copper corosion in chloride solutions by amino-mercapto-thiadiazol and methyl-mercapto- thiadiazol: an impedance spectroscopy and a quantum-chemical investigation[J]. Electrochimca Acta, 2004, 49(17/18): 2761-2770.
[10] 赵 岩, 林昌健, 李 彦, 王景润. 覆铜板在NaCl溶液中腐蚀电化学行为[J]. 物理化学学报, 2007, 23(9): 1342-1346.
ZHAO Yan, LIN Chang-jian, LI Yan, WANG Jing-run. Electrochemical corrosion behavior of copper clad laminate in NaCl solution[J]. Acta Physico-Chimica Sinica, 2007, 23(9): 1342-1346.
[11] OGUZIE E E, OKOLUE B N, EBENSO E E, ONUOHA G N, ONUCHUKWU A I. Valuation of the inhibitory effect of methylene blue dye on the corrosion of aluminium in hydrochloric acid[J]. Materials Chemistry and Physics, 2004, 87(2): 394-401.
[12] MA Hou-yi, ZHAO Shi-yong, CHEN Shen-hao, LIU Xiang-qian. A study of corrosion behavior of copper in the acidic solutions containing cetyltrimethylammonium bromide[J]. Journal of the Electrochimca Society, 2001, 148(5): 482-488.
[13] 罗正贵, 闻荻江. 铜的腐蚀及防护研究进展[J]. 武汉化工学院学报, 2005, 27(2): 17-21.
LUO Zheng-gui, WEN Di-jiang. Research development of corrosion and protection for copper[J]. Journal of Wuhan Institute of Chemical Technology, 2005, 27(2): 17-21.
[14] 曲文娟, 杜荣归, 林昌健. EDA对铜在稀盐酸中的缓蚀效果及硫离子的影响[J]. 电化学, 2006, 12(1): 50-54.
QU Wen-juan, DU Rong-gui, LIN Chang-jian. Effects of sulfide ions on the inhibiting efficiency of EDA as a copper corrosion inhibitor in dilute HCI solution[J]. Electrochemistry, 2006, 12(1): 50-54.
[15] OTMACIC H, TELEGDI J. Protective properties of an inhibitor layer formed on copper in neutral chloride solution[J]. Journal of Applied Electrochemistry, 2004, 34(5): 545-550.
基金项目:重庆市自然科学基金资助项目(CSTC-2004BA4024)
收稿日期:2009-03-10;修订日期:2009-05-18
通信作者:黄文章,副教授,博士;电话:023-65023753;E-mail: hwz4889@163.com
(编辑 李艳红)