
Hydro/solvo-thermal synthesis of ZnO crystallite with particular morphology
LI Yan(李 酽), LIU Chuan-sheng(刘传生)
College of Science, Civil Aviation University of China, Tianjin 300300, China
Received 19 April 2008; accepted 15 December 2008
Abstract: ZnO with controllable morphologies of hexangular pillars, tubes, tablets and tube-structured needles was hydro/ solvo-thermally synthesized by simply changing solvents among H2O, H2O2, alcohol and olefin, using Zn(NO3)2?6H2O and NaOH as the starting materials. The products were characterized by X-ray diffraction, scanning electron microscopy and photoluminescence (PL) measurement. The growth mechanism of differently structured ZnO was discussed. The results reveal that the synthesized ZnO crystals possess a wurtzite structure, the solvent kind has very different influence on the morphology of ZnO, and the hexangular pillars, tablets, short pillars and needles of ZnO are easily obtained in solvent of H2O, C2H5OH+H2O, wet olefin, and H2O2.
Key words: zinc oxide; synthesis; morphology; solvent; photoluminescence
1 Introduction
Zinc oxide(ZnO), a wide-gap compound Ⅱ-Ⅵsemiconductor with a direct band gap of about 3.4 eV at room temperature and a large exciton binding energy of about 60 meV, has numerous applications, such as photonic devices, transparent conductors, solar cell windows, surface acoustic devices and gas sensors[1-2]. Thus, various synthesis methods have been employed to control particle morphology of ZnO, as size and shape can influence various properties[3-7]. Hexagonal ZnO nano-tetra-pods, nano-rods and nano-pyramid have been synthesized by a thermal evaporation of Zn powder under simultaneous flow of oxygen and argon gases in two-zone furnace in two different temperature regions [8-9]. XU et al[10] grew a single-crystalline ZnO nano-wire on zinc-plated-steel substrate using a flame- synthesis method. Nano-plates, flower-like nanostructure of ZnO were successfully synthesized by employing ZnSO4?7H2O and NaOH as the starting materials at 120 ℃ under hydrothermal condition[11]. BAN et al[12] synthesized ZnO crystals in aqueous solutions containing zinc ions and the morphology of ZnO crystals was examined. It was found that the shape of ZnO crystals changed from a short asymmetric column with a hexagonal flat edge and a rounded one, through a rocket-like shape formed by intergrowth, to a hexagonal rod, as increasing the TMAOH/Zn ratio. A two stage chemical deposition(TSCD) technique[13] was used to produce ZnO films on different substrates from an aqueous solution of zinc complex. The effects of the substrate material on the morphology of the deposited layer were investigated. However, complex procedures, sophisticated equipment, rigid experimental conditions or a low yield were involved in above methods. Large-scale use will require the development of simple, low-cost approaches to the synthesis of ZnO. To our knowledge, a little work has been done to produce a simple synthesis method that is morphologically tunable on ZnO synthesis only by changing solvents. Here we report a straightforward solution method to prepare hexangular pillars, tubes, tablets and tube-structured needles, some new morphology for zinc oxide, by only varying solvent. The products are characterized by X-ray diffraction(XRD) and scanning electron microscopy (SEM). The effect of morphology on the photoluminescence(PL) properties is also investigated.
2 Experimental
2.1 Materials
All reagents of nitrate hexahydrate (Zn(NO3)2·6H2O), sodium hydroxyl (NaOH), hydrogen peroxide (H2O2, 30%) and N,N,N-trimethyl-1- hexadecanaminium bromide (CTABr, C19H42BrN), absolute alcohol (C2H5OH) and wet olefin were analytical-grade, and purchased from commercial market without further purification.
2.2 Preparation of samples
The ZnO crystalline powder was prepared according to the following process. The aqueous solution (0.1 mol/L) of zinc nitrate hydrate and the solution (0.2 mol/L) of sodium hydroxyl were all prepared with deionized water. The sodium hydroxyl solution was added into zinc nitrate solution drop by drop at room temperature under vigorous stirring, which resulted in the formation of a white suspension. The suspension was separated with a centrifuge and washed three times with deionized water, and then washed with absolute alcohol at last. The separated powder was dried at 60 ℃ for 24 h in an oven to obtain the precursor. Subsequently, 3 g precursor materials and 0.01 g CTABr were completely added into 30 mL H2O, 30 mL absolute alcohol, the mixture of 20 mL absolute alcohol and 10 mL H2O, 30 mL wet olefin, 30 mL H2O2, respectively, and the mixture was stirred vigorously for 30 min and then sealed into Teflon-lined autoclaves with a filling capacity of about 35%. The autoclave was treated thermally at 180 ℃ for 72 h. Afterwards, the resulting white precipitate was washed with distilled water and alcohol several times to obtain ZnO crystallites.
2.3 Characterization
The morphology of ZnO particles was investigated by 1530VP model field emission scanning electron microscope(SEM). X-ray diffraction(XRD) of the obtained particles was identified on DX-2000 X-ray diffractometer with Cu Kα radiation (λ=0.154 2 nm). Photoluminescence(PL) spectra of ZnO nanocrystals were measured with a WGY-10 fluorescence spectrophotometer with a Xe lamp (150 mW). The excitation wavelength was fixed at 325 nm. The emission spectrum of solid zinc oxide powder samples at room temperature was observed in the wavelength range of 350-600 nm.
3 Results and discussion
3.1 XRD analysis of specimen
The XRD patterns of the synthesized ZnO particles in different solvents of H2O, H2O2, alcohol and olefin, being completely uniform, can match the standard bulk wurtzite structure (P63mc), as shown in Fig.1. The diffraction peaks can be well indexed to wurtzite ZnO with lattice parameters of a=0.324 982 nm and c= 0.520 661 nm (JCPDS Card No.36-1451). The high intensity of all diffraction peaks indicates a good crystallinity of the synthesized ZnO under these experimental conditions. It is obviously that ZnO crystal can be fully grown in H2O, H2O2, alcohol or olefin medium.
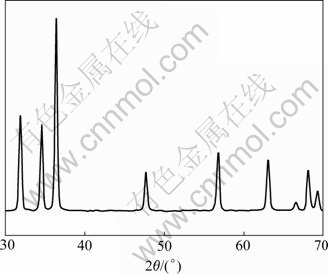
Fig.1 XRD pattern of synthesized ZnO
3.2 Morphology and growth mechanism of ZnO
SEM images of the prepared samples are shown in Fig.2 and Fig.3. In Fig.2(a), the sample prepared in water shows hexangular pillars with a diameter of 200 to 300 nm and a length ranging from 400 to 500 nm, and the aspect ratio is 2 to 3. Fig.2(b) shows the samples prepared in alcohol alone, which yields hexangular tube structure. The hexangular tubes possess a diameter of 100 to 200 nm and a length of 400 to 500 nm, which derive from the strain forming during further growth and self-assemble approach of smaller particles. Fig.2(c) gives a general overview of the products synthesized in olefin, which are hexangular pillar, containing holes on their surface. Fig.2(d) shows the hexangular tablets prepared from mixed solvent of water and alcohol with a bulk ratio of 0.5, with thin and even taper, and a diameter range of 1-2 ?m and a thickness of 200 nm. The needle shape of the sample synthesized in H2O2 is shown in Fig.3, in which the spiral, tubes, and honey comb structure are found.

Fig.2 SEM images of ZnO particles synthesized at 180 ℃ in H2O (a), alcohol (b), wet olefin (c) and mixture of H2O and alcohol (d)

Fig.3 SEM images of ZnO particles synthesized at 180 ℃ in H2O2
On the basic of above results, the growth mechanism of different structure ZnO can be discussed as follows. The polar crystal of ZnO nanostructure grows preferentially along [0001] direction (+c axis terminated by zinc) because of the lowest surface energy of (0002) facet. The growth velocity along <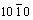 > directions is slower than that along
> directions is slower than that along 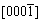 direction, so that the hexangular pillar morphology is often obtained. Meanwhile, the growth of ZnO along [0001] direction
direction, so that the hexangular pillar morphology is often obtained. Meanwhile, the growth of ZnO along [0001] direction
may be accelerated or suppressed in solvo or hydro-thermal system as having or lacking oxygen and hydroxyl OH-. The tablets can be produced by suppressing the growth along +c axis under certain conditions. Short hexangular pillar and tablets structures can be achieved in solvent of alcohol lacking hydroxyl OH-. A loose hexangular pillar structure of ZnO can be achieved by a quick growth under high temperature in our experiment. In addition, the strain, induced by the inner shrink of ZnO rods during its overgrowth for long time (72 h), causes a collapse or crack between the surface layer and the inner of ZnO rods, which prompts the ZnO rods to be tubes. The growth mechanism of ZnO in H2O2 is considered similar with the viewpoint expressed in Ref.[14], an initially grown sheet turns into a roll and the cone-shaped roll transforms into a cylindrical tube, presumably via a dislocation “zipper” mechanism. Finally, a hollow tube transforms into a needle. Crystal morphology is determined by the relative growth rates of the crystal planes, which can differ greatly due to differences in surface free energies[15]. In our system, other reagents were kept indeclinable, and the solvent was changed. The results indicate that the different solvent plays significant roles in stabilizing specific crystallographic planes of ZnO crystal. It is found that, the hexangular pillar forms very easily in the aqueous solution, in which the forming of [ZnO4]4- tetrahedron is prompted and the growth along c-axis is accelerated. In solvent of H2O2, the growth along c-axis and some side faces is synchronously enhanced due to H2O2 molecule and abundant oxygen atoms, which results in the forming of tube-structure needles. Being lack of hydroxyl OH- in alcohol, the growth along c-axis is greatly restrained, which results in very thin hexangular tablets (Fig.2(c)). Wet olefin, a nonpolar solvent different from H2O, alcohol and H2O2, has the same effect on different faces of ZnO, which causes a hexangular pillar shape with aspect ratio of one.
3.3 Photoluminescence property of specimen
The room temperature photoluminescence spectra of the as-prepared ZnO crystallites fabricated at 180 ℃ for 72 h were recorded, as shown in Fig.4. A broad blue emission at about 448.5 nm and a strong green emission peak centered at 554 nm are observed for all samples. Fig.4(c) shows that the green emission peak of ZnO tablet in Fig.2(c) is obviously lower than that in other shapes. ZnO needle (Fig.3) synthesized in H2O2 shows the weak violet peak at 399.9 nm (Fig.4(e)). Moreover, several scattered lower and wider peaks, from violet emission excitation, can be found between blue and green emission peaks. It can be seen that the room temperature photoluminescence spectra of ZnO synthesized in different solvent possess a similar shape and a slight intensity difference.
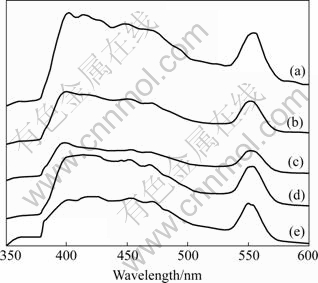
Fig.4 Room temperature photoluminescence spectra of ZnO samples synthesized in H2O (a), alcohol (b), mixture of H2O and alcohol (c), wet olefin (d) and H2O2 (e)
4 Conclusions
1) Zinc oxide morphology of hexangular pillar, tube, tablet and tube-structured needle, can be efficiently controlled by changing solvents among H2O, H2O2, alcohol and olefin.
2) Hexangular pillar of ZnO is very easily developed in aqueous solution, and the growth along c-axis and some side face is synchronously enhanced in H2O2, resulting in the forming of tube-structure needles.
3) Wet olefin, a nonpolar solvent, can cause a hexangular pillar shape with aspect ratio of one.
4) XRD results show that the synthesized ZnO crystals possess a wurtzite structure. Compared with the sample prepared in solvents of H2O, alcohol and olefin, the violet peak is not observed in sample synthesized in H2O2 because the near-band-edge structure may be changed by an oxygen-rich environment.
References
[1] UMAR A, RAHMAN M M, KIM S H, HAHN Y B. Zinc oxide nano-nail based chemical sensor for hydrazine detection [J]. Chem Commun, 2007(2): 166-168.
[2] WAMA R, UTIYAMA M, PLASHNITSA V V, MIURA N. Highly sensitive impedance-based propene sensor using stabilized zirconia and zinc oxide sensing-electrode [J]. Electrochem Commun, 2007, 9(12): 2774-2777.
[3] WANG Q X, YOU L P, ZHANG X Z, WANG R M, LV Y Z, GUO L. Single-crystal star-like zinc oxide: Synthesis, characterization and growth mechanism [J]. Rare Metals, 2006, 25: 193-199.
[4] POKROPIVNY V V, KASUMOV M M. Synthesis and growth mechanism of zinc oxide nanostructures in arc discharge [J]. Tech Phys Lett, 2007, 33(1): 44-47.
[5] XIE X H, LI X J, YAN H H. Detonation synthesis of zinc oxide nanometer powders [J]. Mater Lett, 2006, 60(25/26): 3149-3152.
[6] LAZARECK A D, CLOUTIER S G, KUO T F, TAFT B J, KELLEY S O, XU J M. DNA-directed synthesis of zinc oxide nanowires on carbon nanotube tips [J]. Nanotechnology, 2006, 17(10): 2661-2664.
[7] WANG H Q, LI G H, JIA L C, WANG G Z, TANG C J. Controllable preferential-etching synthesis and photocatalytic activity of porous ZnO nanotubes [J]. J Phys Chem C, 2008, 112(31): 11738-11743.
[8] SINGH J, TIWARI R S, SRIVASTAVA O N. Synthesis of zinc oxide nanotetrapods and nanorods by thermal evaporation without catalysis [J]. Nanosci Nanotechnol, 2007, 7(6): 1783-1786.
[9] CHEN H S, QI J J, ZHANG Y, ZHANG X M, LIAO Q L, HUANG Y H. Controlled growth and field emission properties of zinc oxide nanopyramid arrays [J]. Applied Surface Science, 2007, 253: 8901-8904.
[10] XU F S, LIU X F, TSE S D, COSANDEY F, KEAR B H. Flame synthesis of zinc oxide nanowires [J]. Chem Phys Lett, 2007, 449(1/3): 175-181.
[11] SHANG T M, SUN J H, ZHOU Q F, GUAN M Y. Controlled synthesis of various morphologies of nanostructured zinc oxide: Flower, nanoplate, and urchin [J]. Cyst Res Technol, 2007, 42(10): 1002-1006.
[12] BAN T, SAKAI T, OHYA Y. Synthesis of zinc oxide crystals with different shapes from zincate aqueous solutions stabilized with triethanolamine [J]. Cryst Res Technol, 2007, 42(9): 849-855.
[13] VAEZI M R, SADRNEZHAAD S K. Effects of substrate material and annealing temperature on morphology of zinc oxide films [J]. Mater Sci Technol, 2006, 22(3): 308-314.
[14] POKROPIVNY V V, KASUMOV M M. Synthesis and growth mechanism of zinc oxide nanostructures in arc discharge [J]. Tech Phys Lett, 2007, 33(1): 44-47.
[15] ANDELMAN T, GONG Y Y, POLKING M, YIN M, KUSKOVSKY I, NEUMARK G, ?BRIEN S. Morphological control and photoluminescence of zinc oxide nanocrystals [J]. J Phys Chem B, 2005, 109(30): 14314-14318.
Foundation item: Project(05yfJMTC 12900) supported by the Natural Science Foundation of Tianjin, China
Corresponding author: LI Yan; Tel: +86-22-24092519; Fax: +86-22-24092514; E-mail: liyan01898@163.com
DOI: 10.1016/S1003-6326(08)60285-X
(Edited by YUAN Sai-qian)