J. Cent. South Univ. Technol. (2011) 18: 1365-1370
DOI: 10.1007/s11771-011-0847-7
Modulating absorption band of triangular silver nanoplates in aqueous solvent and on substrates using tannin as reducing agent
YI Zao(易早)1, 2, NIU Gao(牛高)1, HAN Shang-jun(韩尚君)1, LUO Jiang-shan(罗江山)1,
CHEN Shan-jun(陈善俊)1, 3, YE Xin(叶鑫)1, YI You-gen(易有根)2, TANG Yong-jian(唐永建)1
1. Research Center of Laser Fusion, China Academy of Engineering Physics, Mianyang 621900, China;
2. School of Physical Science and Technology, Central South University, Changsha 410083, China;
3. Institute of Atomic and Molecular Physics, Sichuan University, Chengdu 610065, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: Triangular silver nanoplates in aqueous solvent and on the surface of quartz substrate have been synthesized by seed-mediated growth approach in the presence of tannin. It was found that both the amount of tannin and the small triangular silver nanoplate seeds added to the growth solution are the key factors to modulation absorption band of triangular silver nanoplates. The optical in-plane dipole surface plasmon resonance (SPR) bands of these Ag nanoplates can be tuned from 608 nm to 980 nm via tannin deoxidization method. The formation mechanism of triangular silver nanoplates was proposed. The tannin deoxidization method realizes a convenient modulation of the absorption band of Ag nanostructures within the visible near-infrared (IR) region both in aqueous solvent and on substrates under mild conditions.
Key words: silver nanoplate; tannin; seed-mediated growth; surface plasmon resonance; near-infrared region
1 Introduction
Synthesis of metal nanoparticles is an interesting field because of their fascinating size- and shape- dependant optical, catalytic, electrical and magnetic properties [1-4]. Silver is a particularly interesting metal to be examined in the nanoscale, due to its unique optical properties. The absorption band tunability of Ag nanoplates promises their potential application in tremendous fields such as optical contrast-enhanced imaging, photothermal cancer therapy, drug release, and glass coating for effective attenuation of infrared solar radiation [5-7]. JANA et al [8] made the in-plane dipolar surface plasmon resonance (SPR) band of Au nanorods shift from 530 nm to 1800 nm via a seed-mediated growth approach. CHEN and CARROLL [9] found that the size-sensitive in-plane dipole plasmon absorption bands of the Ag nanoplates can be shifted to ~1 000 nm in the near-infrared (IR) region. At that time, the aspect ratio of nanoplates reached 9, opening new possibilities for various near-IR related applications. By controlling the pH, XUE and MIRKIN [10] made the SPR bands of silver nanoprisms span to the visible and near-IR regions of the spectrum. Hereinbefore the modulating of SPR absorption band is successful only in aqueous solvent phase. However, few works have been done on substrates. ASLAN et al [11] reported the deposition of silver triangular nanoplates on conventional glass substrate which is based on the seed-mediated CTAB-directed growth of silver triangles on glass surfaces, and the in-plane dipole plasmon absorption bands of the nanoplates can barely be shifted to 600 nm.
In this work, seed-mediated growth strategy was employed to modulate the SPR absorption of silver triangular nanoplates from 600 nm to 918 nm via tannin as a mild reducer in aqueous solvent and on substrates. All experiments were conducted at room temperature. The size and shape of the silver particles could be controlled by varying the experimental parameters such as tannin concentration and the quantity of seed solution. Through comparing the SEM images and SPR absorption of the products, the difference in the growth mechanism between tannin as a mild reducer in aqueous solvent and on substrates was analyzed.
2 Experimental
Triangular Ag nanoplate seeds were prepared by dual-reduction method reported by M?TRAUX and MIRKIN [12]. The reaction mechanism of triangular Ag nanoplate seeds was investigated by careful spectroscopy characterization [13].
The as-prepared Ag colloid was continually stirred for one day to degrade the excess of H2O2, and then silver triangular nanoplates grew up by tannin deoxidization. The typical synthesis was as follows: 0.05 mL, 0.2 mL and 0.6 mL Ag colloid were added into three bottles of 3.1 mL mixture containing 0.03 mol/L AgNO3, 6 mmol/L tannin and 0.03 mol/L sodium dodecyl sulfate (SDS), respectively. Then, the solutions were stirred for about 10 min. These solutions were stored in a darkroom at room temperature. The mixture in the bottles was muddy, and on the bottom of the bottles appeared some sediments. After 24 h, the colour of three solutions became silver gray, purple and green, respectively.
Quartz substrates (1 cm × 1 cm) were prepared by silanized method reported by ASLAN et al [11]. APS-coated quartz substrates were immersed in the silver seed solution for 2 h, rinsed with deionized water, and then dried in a stream of nitrogen gas. In the reduction process, the growth solution contained 50 mL of 0.01 mol/L AgNO3, 50 mL of 1.5 mmol/L tannin, 5 mL of 0.01 mol/L sodium dodecyl sulfate and 300 mL of deionized water. And the aqueous solution was separated into three portions: 40.5 mL (I), 81 mL (II) and 121.5 mL (III). Then, the silver-seed-coated quartz substrates were immersed in these aqueous solutions and deposited in darkroom at room temperature for 24 h.
The images of transmission electron microscopy (TEM) were obtained by a JEM-2010 microscope using an accelerating voltage of 120 kV and the samples were prepared by placing a drop of aqueous dispersion products on carbon-coated copper grid. The products were identified by X-ray diffraction (XRD, X’Pert PRO) with Cu Kα radiation.
The optical properties of these nanoparticles were measured by a Perkin-Elmer Lambda 12 spectro- photometer. The scanning electron microscopy (SEM) images were recorded using a Leica Cambridge S440 field emission scanning electron microscope with an accelerating voltage of 5 kV.
3 Results and discussion
3.1 TEM images of triangular silver nanoplates in aqueous solvent
TEM images of the initial Ag nanoparticles and the products by seed-mediated growth approach in aqueous solvent are shown in Fig.1. The initial particles obtained by dual-reduction method are in shape of triangle with mild outlines, and their average edge length is measured to be (35±8) nm (Fig.1(a)). The inset of Fig.1(a) shows the close stacking of several nanoplates with the lateral sides upward, enabling us to estimate the thickness of these nanoplates to be about 5.3 nm. Figures 1(b)-(d) show the TEM images of products deoxidized by tannin in aqueous solvent with different volumes of seed solution for 24 h. When 0.6 mL seed solution is introduced, the size of silver nanoplates in the range of 80-120 nm is obtained (Fig.1(b)). When the volume of the seeds solution is decreased to 0.2 mL (Fig.1(c)), the size of the nanoplates increases to 110-170 nm. Further the seed solution is decreased to 0.05 mL (Fig.1(d)), resulting in the formation of larger nanoplates with size of 180- 210 nm. When silver ions in the growth solution are kept at a constant, more nucleation centers will evidently lead to fewer silver ions for a single nanoplate. So, it is reasonable that the size of the nanoplates will decrease with increasing the amount of seeds. The inset in Fig.1(c) is the XRD pattern of the products. The three peaks are assigned to the diffraction of (111), (200) and (220) planes of FCC silver. The cell parameter is calculated from this pattern to be 4.084 ? which is in agreement with the standard card (PCPDF No.04-0783). It is noticed that the ratio between the intensities of (111) and (200) diffraction peaks is much higher than the conventional value (13 versus 4). This indicates that the nanoparticles are (111)-oriented, and tend to lay with these planes parallel to the supporting substrate. Thus, diffraction intensity of the (111) plane should be greatly enhanced compared to that of other planes. From the structure analyses on the XRD pattern, it is clear that the products are nanoplates with (111) facets as the basal planes [14-15].

Fig.1 TEM images of products: (a) Triangular Ag nanoplate seeds (Inset: plates stacked); (b)-(d) Products deoxidized by tannin in solution with different volumes of seed solution at 24 h: (b) 0.6 mL; (c) 0.2 mL; (d) 0.05 mL; (Inset of in Fig.1(c): OPML-XRD pattern of product)
3.2 UV-Vis absorption spectra of triangular silver nanoplates in aqueous solvent
The UV-Vis absorption spectra of triangular silver nanoplates in aqueous solvent are shown in Fig.2(a). Absorption spectra of these products normally exhibit several peaks: a small peak at around 332 nm is attributed to the out-of-plane quadrupole resonance of the nanoplates; the second peak at the longest wavelength side, which is very sensitive to the size and aspect ratio of the particles, is due to the in-plane dipole plasmon resonance. Fig.2(a) reveals that the seeds of Ag nanoplates exhibit three peaks: a small peak at 332 nm, a shoulder peak at 453 nm, and a strong peak at 608 nm, which can be ascribed to the out-of-plane quadrupole resonance, out-of-plane dipole resonance and in-plane dipolar SPR absorption band of Ag nanoplates, respectively [16]. The in-plane dipolar band is always paid more attention, as it holds the strongest peak and is most sensitive to the changes of the anisotropy and component of the nanoplates [17]. These peaks red shift because the size of the plates increases, according to Fig.2(a). When the volume of seed solution is 6 mL, the other two peaks at 442 nm and 674 nm are assigned to the out-of-plane dipole resonance and in-plane dipolar SPR band. The shoulder peak at 415-453 nm is normally attributed to the out-of-plane dipole resonance of the nanoplates. However, its relative intensity is much stronger than the theoretically expected value. Since spherical silver particles may also have absorption band in this range, it may imply the existence of spherical particles in the aqueous solvent. With the seed solution volume being decreased to 2 mL, the two peaks red shift to 450 nm and 800 nm, respectively, which is consistent with the size increase of the triangular nanoplates [18]. The in-plane dipolar band red shifts with increasing the plate size, and it could even reach 980 nm in the near-IR region when the volume of seed solution is 0.05 mL.
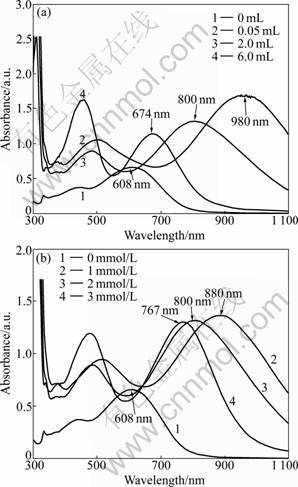
Fig.2 UV-Vis absorption spectra of triangular silver nanoplates: (a) Products obtained from different amounts of seeds; (b) Products obtained from different tannin concentrations
Figure 2(b) displays the absorption spectra of the products obtained with different tannin concentrations. In the absence of tannin, the UV-Vis absorption spectra of triangular silver nanoplates keep immovability, indicating that tannin is necessary for silver nanoplates growth. As the concentration of tannin is minished from 3 mmol/L to 1 mmol/L, the in-plane dipolar SPR absorption band of three products red shift to 767 nm from 800 nm. Slow deoxidization reaction and the crystallization process are advantageous to the anisotropic nano-structured particles. When the aspect ratio of triangular nanoplates is increased, the in-plane dipolar band will red shift, while the loss of corner sharpness of the triangles will make the band blue shift. The blue shifting reveals that during the growth process, loss of the corner sharpness leads to an overwhelming predominance over the increase of the aspect ratio, thus resulting in the observable blue shift of the in-plane dipolar band. The change of the in-plane dipolar absorption band indicates that tannin is necessary for silver nanoplates formation.
3.3 SEM images of products on substrates
Figure 3 presents the SEM images of the products on substrates with different volumes of growth solution for 24 h. Figure 3(a) shows the products in solution I. The size of the nanoplates increases to the range of 110- 170 nm, and small spherical particles can be found. It should be pointed out that secondary homogenous nucleation occurs here. Thereby these small particles can be found though they are barely found in silver seed solutions. The amount of silver ions increases with the growth solution volume increasing, so as to the size of the nanoplates, as to crowd on substrates. When the volume of growth solution is 81 mL, the average size of the nanoplates increases to 200 nm. Some secondary homogenous nucleation particles lay over on top of the particles grown on the first layer (Fig.3(b)). In Fig.3(c), more particles lay over on the first layer, and the particles arrange very closely.
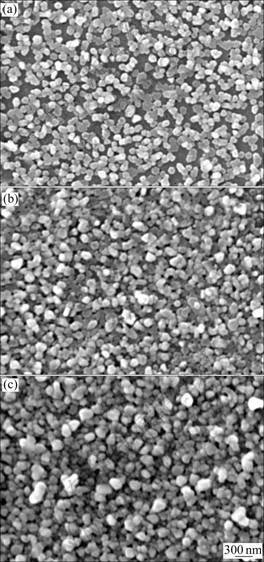
Fig.3 SEM images of products on substrates with different volume of growth solution for 24 h: (a) 40.5 mL; (b) 81 mL; (c) 121.5 mL
3.4 AFM images of products on substrates
Figure 4 shows the AFM images of the products on substrates with different volumes of growth solution for 24 h. It can be clearly seen that all particles have grown substantially, and the height of the particles layer increases. As seen from Fig.4(a), when the volume of growth solution is 40.5 mL, the height of the nanoplates is 40-120 nm. The majority of particles is silver triangular nanoplates, and some spherical nanoparticles can be found. As shown in Fig.4(b), the height of the nanoplates is 60-180 nm when the volume of growth solution increases to 81 mL. More spherical nanoparticles appear. Figure 4(c) shows that the height of the particles increases as the volume of growth solution increases to 121.5 mL. The silver triangular nanoplates of the first layer are not found because more spherical nanoparticles lay over on the first layer.
3.5 UV-Vis absorption spectra of triangular silver nanoplates on substrates
Figure 5(a) displays the absorption spectra of the products on substrates with different volumes of growth solution for 24 h. The SPR absorption band of metal nanoparticles is produced by the particles of collective oscillation that the surface particles of conduction band electrons are driven by photoelectric field. The peak absorption is affected by the morphology and scale of the particles, the dielectric constant of the medium, the characteristic of particle surface-coupled molecules, the degree of aggregation of particles and other factors [19]. As a result, there are much distinctness in the absorption spectra of the products in Fig.5(a) and Fig.2(a). The in-plane dipolar SPR absorption band will red shift when the volume of growth solution increases. As the volume of solution increases, the coupling peaks rise and red shift from 633 nm to 980 nm. Because the size of the nanoplates increases with the volume of growth solution increasing, the gap between the particles becomes narrow and particles arrange more closely. And the secondary particles lay on the first layer, forming a localized high-density particle aggregation. These reasons accelerate inter-particle dipole coupling growth, which can also be verified from the above SEM pictures.
The growth of the products was monitored by UV-Vis spectroscopy, and the SPR absorption bands of products with different reaction time were exhibited, as shown in Fig.5(b). The SPR absorption band of the surface of silver-seed-coated quartz substrates (0 h) is not obvious, which can be attributed to the very low density of seeds on the substrates surface. The absorbance of absorption band increases with time increasing in growth solution. This indicates that the amount of deoxidized Ag increases as the time in growth solution increases, resulting in the film of silver nanoparticles becoming thicker on substrates. As the reaction proceeds to 24 h, the position of the in-plane dipolar SPR absorption band red shifts gradually. And at the same time, the shoulder peaks become stronger, indicating that the size of the silver spherical nanoparticles increases. After the reaction proceeds to 24 h, the position of the in-plane dipolar SPR absorption band blue shifts slowly, and the shoulder peaks are stronger, because some superfluous tannin makes the particles contact with each other and Ostwald reaction occurs. The atoms of small particles transfer to large particles through solution as medium, resulting in the proportion of large particles increases and the average size of the particles fills out. So, the shoulder of 500- 600 nm peaks moves higher and the in-plane dipolar SPR absorption band becomes blue shifted [20].
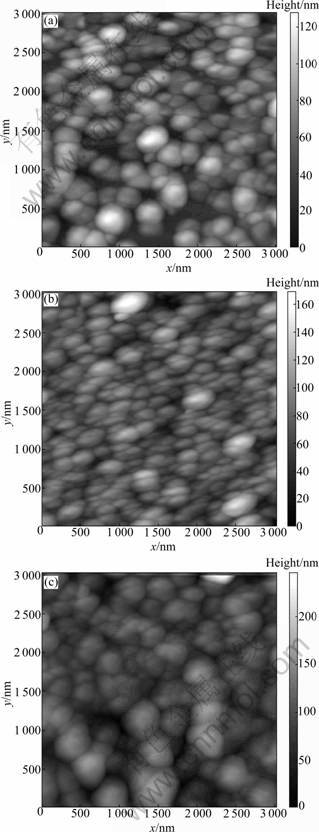
Fig.4 AFM images of products on substrates with different volumes of growth solution for 24 h: (a) 40.5 mL; (b) 81 mL; (c) 121.5 mL
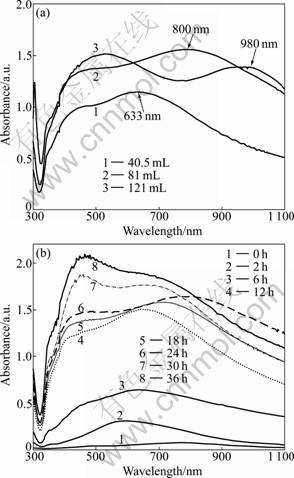
Fig.5 UV-Vis absorption spectra of products at 24 h: (a) Different volume of solution; (b) Different reaction time
Comparing the SEM and SPR absorption of the products in the aqueous solvent and on the substrate, it is found that the growth mechanism of tannin as a mild deoxidization in aqueous solvent and on the substrates is different. In aqueous solvent phase, spherical particles are less than those on the solid phase. This is because in aqueous solvent phase, the secondary nucleation of spherical particles will activate Ostwald reaction and become small particles very easily, then these small particles will transform to triangular Ag nanoplate by the role of tannin. However, when triangular Ag nanoplate seeds are fixed on substrate, the secondary nucleation of the particles will become big spherical particles directly by tannin deoxidization. Therefore, almost all of the particles are triangle in aqueous solvent, but the yield of triangle particles is very low, while many spherical particles appear on the substrate under the same conditions.
4 Conclusions
1) The amount of seeds, the concentration of tannin and the volume of reaction solution play important role on the morphology and the SPR absorption of the products of silver nanoplates. The change of the in-plane dipolar absorption band indicates that tannin is necessary for silver nanoplates growth.
2) The synthetic strategy may provide a convenient way to synthesize other noble metals with planar structures and give an idea to tune the in-plane dipolar absorption band of noble metals. Furthermore, in-situ growth of silver nanoplates nanostructure can form a localized high-density particle aggregation on quartz substrates. This method can be applied to other substrates. And through comparing the SEM and SPR absorption of the products in the aqueous solvent and on the substrate, it is found that the growth mechanism of tannin as a mild deoxidization in aqueous solvent and on substrates is different.
References
[1] PEREZ J J, SANTOS I P, MARZAN LM, PAUL M. Gold nanorods: Synthesis, characterization and applications [J]. Coordin Chem Rev, 2005, 249: 1870-1901.
[2] KELLY K L, CORONADO E, ZHAO L L, GEORGE C S. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment [J]. J Phys Chem B, 2003, 107(3): 668-677.
[3] TIAN Z Q, REN B, WU D Y. Surface-enhanced raman scattering: From noble to transition metals and from rough surfaces to ordered nanostructures [J]. J Phy Chem B, 2002, 37(106): 9463-9483.
[4] DEL C T, AROCA R D S, RODRIGUEZ J A. Langmuir-blodgett mixed films of titanyl(IV) pthalocyanine and arachidic acid: Molecular orientation and film structure [J]. Langmuir, 2003, 19(9): 3747-3751.
[5] KALELE S, GOSAVI S W, URBAN J, KUIKIARNI S K. Nanoshell particles: Synthesis, properties and applications [J]. Curr Sci (India), 2006, 91(8): 1038-1052.
[6] CHEN J, WILEY B, LI Z, CAMPBELL D, SAEKI F, CAN G H, AN L, LEE J, LI X, XIA Y. Gold nanocages engineering: Their structure for biomedical applications [J]. Adv Mater, 2005, 17(18): 2255-2261.
[7] SHANKAR S S, RAI A, AHMAD A, MURALI S. Controlling the optical properties of lemongrass extract synthesized gold nanotriangles and potential application in infrared-absorbing optical coatings [J]. Chem Mater, 2005, 17(3): 566-572.
[8] JANA N R, GEARHEART L, MURPHY C J. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template [J]. Adv Mater, 2001, 13(18): 1398-1393.
[9] CHEN S H, CARROLL D L. Silver nanoplates: Size control in two dimensions and formation mechanisms [J]. J Phys Chem B, 2004, 108(18): 5500-5506.
[10] XUE C, MIRKIN C A. pH-switchable silver nanoprism growth pathways [J]. Angew Chem, 2007, 119(10): 2082-2084.
[11] ASLAN K, LAKOWICZ J R, GEDDES C D. Rapid deposition of triangular silver nanoplates on planar surfaces: Application to metal-enhanced fluorescence [J]. J Phys Chem B, 2005, 109 (13): 6247-6251.
[12] M?TRAUX G S, MIRKIN C A. Rapid thermal synthesis of silver nanoprisms with chemically tailorable thickness [J]. Adv Mater, 2005, 17(4): 412-415.
[13] GUO Bin, TANG Yong-jian, LUO Jiang-shan, CHENG Jian-ping. Study on absorption and emission spectroscopy of triangular silver nanoplates prepared by dual reduction method [J]. Precious Metals, 2008, 29(2): 5-10 (in Chinese)
[14] MAILLARD M, HUANG P, BRUS L. Ag nanodisk growth by surface plasmon enhanced photoreduction of adsorbed [Ag+] [J]. Nano Lett, 2003, 3(11): 1611-1615.
[15] CHEN S, CARROLL D L. Silver nanoplates: Size control in two dimensions and formation mechanisms [J]. J Phys Chem B, 2004, 108(18): 5500-5506.
[16] KELLY K L, CORONADO E, ZHAO L L, GEORGE C S. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment [J]. J Phys Chem B, 2003, 107(3): 668-677.
[17] JIN R C, CAO Y W, MIRKIN C A. Photoinduced conversion of silver nanospheres to nanoprisms [J]. Science, 2001, 294(30): 1901- 1903.
[18] MILLSTONE J E, METRAUX G S, MIRKIN C A. Controlling the edge length of gold nanoprisms via a seed-mediated approach [J]. Adv Funct Mater, 2006, 16(10): 1209-1214.
[19] YI Zao, TANG Yong-jiang, YI You-gen, LI Kai, LUO Jiang-shan, LI Xi-bo, ZHANG Jian-bo, YE Xin. Preparation of hollow silver microspheres and their characterization [J]. High Power Laser and Particle Beams, 2009, 21(9): 1354-1359. (in Chinese)
[20] JIANG X C, YU A B. Silver Nanoplates: A highly sensitive material toward inorganic anions [J]. Langmuir, 2008, 24(8): 4300-4309.
(Edited by HE Yun-bin)
Foundation item: Project(10804101) supported by the National Natural Science Foundation of China; Project(2007CB815102) supported by the National Basic Research Program of China; Project(2007B08007) supported by the Science and Technology Development Foundation of Chinese Academy of Engineering Physics
Received date: 2010-01-18; Accepted date: 2011-03-24
Corresponding author: TANG Yong-jian, Professor; Tel: +86-816-2484233; E-mail: myyz1984@yahoo.cn