Article ID: 1003-6326(2005)05-1138-07
Sulphidization flotation for recovery of lead and zinc from oxide-sulfide ores
FA Keqing1, Jan D.Miller1, JIANG Tao(姜 涛)2, LI Guang-hui(李光辉)2
(1. Department of Metallurgical Engineering, University of Utah, Salt Lake City, Utah 84112, USA;
2. School of Resources Processing & Bioengineering, Central South University,Changsha 410083, China)
Abstract: A new flowsheet was developed to recover the valuable minerals from oxide or oxide-sulfide ores of lead and zinc. The flowsheet consisted of flotation of sulfide minerals, desliming and sulphidization-flotation of oxide minerals. The corresponding reagent system and techniques to the flowsheet were investigated. Batch and continuous tests show that the dosage of sodium sulfide, temperature, and collector type are main affecting factors on the recovery of smithsonite and cerussite. For the flotation of cerussite, there is an appropriate dosage of sodium sulfide at which the recovery reaches its maximum value. The required sodium sulfide for smithsonite flotation is higher than that for cerussite and the recovery of smithsonite flotation increases with the dosage of sodium sulfide at low level and becomes insensitive at high dosage. The appropriate temperature for smithsonite and cerussite flotation is found to be 25-40℃. Amines are found to be the effective collectors for the flotation of smithsonite after sulphidization. Investigation also shows that desliming prior to sulphidization-flotation is essential to the effective recovery of smithsonite and cerussite, and the desliming process of two-stage hydrocyclon is well feasible and effective for the treatment of lead-zinc oxide ores. A further treatment on the cerussite flotation concentrate by shaking table is proposed to obtain higher lead grade.
Key words: lead; zinc; oxide; flotation; sulphidization CLC number: TD923
Document code: A
1 INTRODUCTION
Smithsonite (ZnCO3) and cerussite (PbCO3) are two typical oxide minerals of lead and zinc. They are semi-soluble type salt minerals with solubility product constants of 1.46×10-10 for ZnCO3 and 7.40×10-14 for PbCO3[1]. Zinc and lead oxide ores are well-known refractory ones because of their inferior floatability and dramatic influence from slimes, which consists of both primary slimes and secondary slimes created during crushing and grinding processes. In order to enhance recovery of the oxidized minerals, a lot of investigations have been done and chelating type reagents are found to be quite effective[2, 3]. In practice, however, semi-soluble oxide minerals are typically recovered by sulphidization-flotation technique[4-9]. Based on the analysis of current technologies in the mineral industry, sulphidization-flotation technique is still a competent and applicable option for the recovery of oxidized lead and zinc minerals.
Sulphidization process converts the oxide mineral surface such as MCO3 (M—metal element) into sulfide surface. This process includes the dissolution of Na2S, in which HS- and S2- ions form in aqueous suspension, adsorption of HS- and S2- ions on mineral surfaces, chemical and/or electrochemical reactions, in which MS forms on the surfaces, and possible dissolution of some surface species. Since the sulfidized surface of oxide minerals is hydrophobic, conventional froth flotation techniques can be used to separate them from the gangue.
The objective of this research is to develop a flexible flowsheet dominated by sulphidization-flotation and investigate corresponding reagent systems and techniques for recovery of lead and zinc from oxide-sulfide ores.
2 EXPERIMENTAL
2.1 Materials
Table 1 lists the main chemical composition of sample used in this research. The sample was taken from Lanping Lead-Zinc Mine, Yunnan Province, China.
The main valuable minerals in the sample are smithsonite, sphalerite, cerussite, gelana, pyrite, anglesite and hemimorphite. The other minerals include calcite, quartz, clay, limonite and hematite. The oxidation degree of zinc minerals is found to be 80%. Among zinc minerals, smithsonite is found to account for 71%.
Table 1 Chemical composition of ore sample used (mass fraction, %)

Mineralogy study also shows that most of zinc and lead minerals appear in fine particles. Some of them are closely associated with limonite, and most of them are disseminated in the gangue minerals independently.
2.2 Methods
Batch flotation tests are performed in a 1.5L cell. 500g of samples, which are ground to desired particle size (80% particles are less than 75μm) prior to flotation, are used for each test.
Continuous flotation tests are conducted in a pilot flotation unit, which has a throughput of 1.2t/d. The run of mine ores, totally about 15t, is first crushed to about 2mm using jaw and roll crushers. The materials are then fully mixed and fed to ball mills. The steps of rough, cleaning and scavenge flotation are calculated previously and tested to fit for desired final recovery and concentrate grade.
It is worthwhile pointing out that desliming is very crucial to the whole system performance. This research uses two-stage hydrocyclone to remove slime, as shown in Fig.1. The underflow of the first cyclone is fed to cerussite flotation circuit. The overflow is sent to the second cyclone as input flow. The overflow of the second cyclone is discarded as slimes. The underflow of the second cyclone is pumped back to the first cyclone as partial feed.
Both batch and continuous tests use the same design to remove slimes.

Fig.1 Desliming system design
3 DESIGN OF FLOWSHEET
Oxide minerals of Pb and Zn often occur more or less with their sulfide minerals. As described above, a part of sulfide minerals of Pb, Zn and Fe are present in the sample. In order to augment the overall profits of mineral processing plant, it is very important to recover as much valuable minerals in the feed as possible. Generally, sulfide minerals have good floatability and should be firstly separated and recovered[10-15]. Slime will seriously interfere with the flotation of oxide minerals and should be removed prior to flotation of oxide minerals. Therefore, in the designed flowsheet, sulfide minerals are first floated in the order of galena, pyrite and sphalerite. The underflow of sulfide flotation circuit is then conducted at the desliming stage for the removal of slime. The deslimed underflow is fed to cerussite flotation circuit to float cerussite. The final step of the flowsheet is the flotation of smithsonite.
The apparent advantage of this flowsheet is that all of the valuable minerals in the ores are recovered. Besides, the desliming operation can remove most of the harmful slime, and increase cerussite and smithsonite recovery. Meanwhile, the desliming operation will remove remaining reagents added in sulfide minerals at flotation stages, which are also harmful to the subsequent sulfidization-flotation operation. If the desliming operation is placed in advance of sulfide mineral flotation, this advantage will not be present.
Continuous flotation tests indicate that it is difficult to obtain sufficiently high cerussite concentrate grade only by froth flotation operation because of the low feed grade of it. Considering the high specific gravity of lead minerals, the flotation concentrate of lead is then fed to a shaking table to obtain the final lead concentrate of high grade in this design.
It is known that the froth mechanical properties are very important in mineral flotation[16]. In the continuous flotation tests, non-polar oil is added in smithsonite flotation circuit. The addition of non-polar oil enhances the hydrophobic characteristic by the collector synergic adsorption and improves the froth mechanical properties. Tests show that the froth becomes clearer and more elastic when non-polar oil is used. Because the load in smithsonite flotation circuit is quite great due to the presence of intermediate circulating load, the improvement of froth properties enhances the whole system performance.
The designed flowsheet for the treatment of lead-zinc oxide-sulfide ores is shown in Fig.2.

Fig.2 Designed flowsheet and reagent system for flotation of oxide-sulfide ores of zinc and lead
4 RESULTS AND DISCUSSION
It is evident that the sulphidization conditions (pH, dosage of sodium sulfide, sulphidization time, temperature, etc) and flotation conditions (pH, type of collectors and their dosage, depressants, etc) are the major affecting parameters on sulphidization-flotation of smithsonite and cerussite. The effects of these parameters will be discussed in this section. The flotation of sulfide minerals is completed with conventional reagents and therefore will not be discussed in details in this paper.
4.1 Effects of sodium sulfide dosage on cerussite flotation
Fig.3 shows the flotation response of cerussite as a function of sodium sulfide dosage. The result is just expected that there is a maximum flotation response of cerussite to the sodium sulfide dosage. At low dosage level of sodium sulfide, the cerussite particle surface is not sufficiently sulfidised. At high dosage, the adsorption of extra S2- ions on the sulfidised film increases with the increase of sodium sulfide and the flotation of cerussite is hence depressed. It is well known that the adding manner of sodium sulfide can greatly affect the flotation performance of cerussite. In this research sodium sulfide is added into the pulp at a 5min interval in batch tests. In continuous tests, the sodium sulfide is added to different sites such as mixing tanks, flotation cells.

Fig.3 Grade and recovery of lead in cerussite flotation concentrate as function of sodium sulfide dosage
In the cerussite flotation circuit, xanthate is used as collector, sodium humate as depressant and sodium hexametaphosphate as dispersant.
4.2 Effects of sodium sulfide dosage on smithsonite flotation
Fig.4 shows batch flotation results. 18-carbon amine is used as the collector. The dispersant and depressant are the same as those used in cerussite flotation. The amount of sodium sulfide required for smithsonite flotation is much higher than that for cerussite flotation. The optimal dosage of sodium sulfide is up to about 3500g/t. This partially results from the higher grade of zinc in the feed. However, the extra S2- ion has not shown a clear depression effect on smithsonite flotation as it has on cerussite. This is in agreement with the results reported by Marabini et al. She found that the smithsonite recovery is not sensitive to the Na2S concentration when the concentration of sodium sulfide is high[17]. The reason for this observation is that at high concentration of Na2S, ZnCO3 component disappears totally and a dense coating of ZnS is formed on the mineral surface. The surface complexes are stable. Essentially, the full formation of ZnS on the surface of Zn oxide minerals results in the amine adsorption less sensitive to the effects of concentration of Na2S. The sulphidization of cerussite is quite different from that of smithsonite. The PbCO3 on mineral surface never disappears completely. Two new species, PbS and PbS2O3, are formed. And only 70%-80% of the surface can be converted to sulfide species. These sulphidization characteristics of cerussite result in an optimal Na2S/Xanthate ratio for the flotation of cerussite.

Fig.4 Grade and recovery of zinc in smithsonite flotation concentrate as function of sodium sulfide dosage
4.3 Effects of pH
The high dosage of sodium sulfide results in a pH range of 10.5-12.0 in the flotation pulp. Flotation results indicate that this pH range is suitable for smithsonite flotation[17]. It is known that amine exists in suspension mainly in the form of RNH2[18] in this pH range. It is expected that the amine can be adsorbed at the smithsonite surfaces through complex bonding, possibly chelating. Generally, commercial sodium sulfide is cheaper than other pH adjusting reagents such as Na2CO3 and NaOH and extra S2- ions in the suspension do not have harmful influence on the flotation of smithsonite, as discussed above. Therefore, a relatively high Na2S concentration is usually used in order to achieve both a high pH value and sufficient sulphidization of smithsonite surface.
4.4 Pulp solid content
It is found that the good flotation performance is obtained when the pulp solid mass concentration is in the range of 35%-40%. Higher content (>40%) is not practical due to the wear of equipment, high consumption of energy, and inferior fluidity of pulp. However, if the content is below 25%, the flotation response is extremely poor no matter how many reagents are used. Such a phenomenon is also observed in phosphate flotation. It is usually believed that in relatively high concent pulp, the concent of reagents is also high so that the adsorption of reagents on mineral surfaces is dynamically favorable and the adsorption density of reagents on surface will be increased. The flotation response is thus enhanced.
4.5 Effects of temperature
Figs.5 and 6 show the temperature effects on the flotation of smithsonite and cerussite. It is evident that both the cerussite and smithsonite flotation recoveries drop very sharply when the pulp temperature is below 20℃. Temperature influence on mineral flotation has been investigated extensively[17, 19, 20]. Marabini et al[17] reported that the sulphidization of smithsonite surface at 40℃ is better than that at 20℃, but if the temperature is increased to 60℃, the sulphidization efficiency is decreased. Massacci et al reported that amine adsorption at smithsonite surface in the presence of low concentration sodium sulfide is an endothermic reaction. However, if the concentration of sodium sulfide is high and pH value exceeds 8.5-9.0, there is a marked decrease in the heat evolved[20]. The fact that the heat evolved decreases indicates that there is a decrease in the amount of adsorbed amine in high sodium sulfide solution. From the literature and results of this research, it may be concluded that it is not applicable to float smithsonite at very high temperature such as at 60℃. However, if the pulp temperature is too low, e.g., below 20℃, the sulfidization and the amine adsorption are not sufficient since the adsorption reactions are endothermic. Thus appropriate temperature for the flotation of cerussite and smithsonite should be 25-40℃.
4.6 Influence of slime and desliming
The slime influence can be immediately seen from Table 2, which shows the comparison of the cerussite flotation test results with and without desliming. The dosage of reagents is the same in both cases except for sodium sulfide. Without desliming, the flotation of cerussite consumes more sodium sulfide and yields a lower grade and recovery of lead than those with desliming operation. The effect of slime on oxide flotation is strong and the reason is complicated[9, 21].

Fig.5 Temperature effects on flotation recovery of smithsonite and cerussite
It turns out that the desliming operation is essential for successful flotation of cerussite and smithsonite. In laboratory, siphon and hydrocyclone desliming methods are tested. Experimental results show that zinc loss in removed slimes is higher and the grade and recovery of it in the concentrate are lower by siphon desliming than those by hydrocyclone desliming.

Fig.6 Effects of temperature on grade of smithsonite and cerussite concentrates
4.7 Continuous flotation results
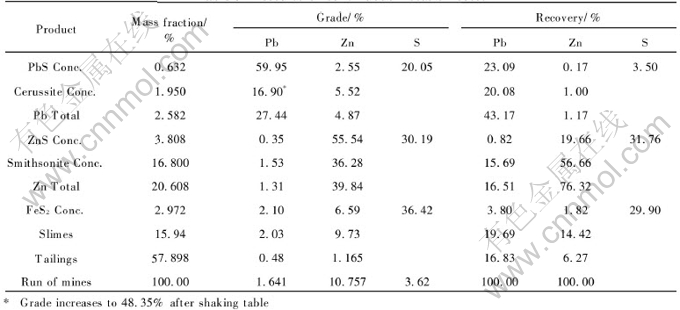
Table 3 lists the results of continuous flota tion with the flowsheet proposed and reagent systems achieved above. Total running time is 104h with a total input of 15t of the ore. The results indicate that the proposed flowsheet is feasible and the reagent system achieved is effective for the treatment and recovery of lead and zinc oxide-sulfide ores.
Table 2 Effect of desliming on cerussite flotation

Table 3 Results of continuous flotation tests
4.8 Mineralogy study of tailings
Mineralogical studies reveal that zinc minerals in the tailing are extremely finely disseminated ZnCO3, which is not liberated from gangues, and/or zinc silicate, which is not able to be recovered by flotation[22, 23]. Overall smithsonite flotation recovery with respect to the total ZnCO3 is estimated to be 80% by comparing the contents of smithsonite in tailing and slimes with those in run of mine. Smithsonite is mainly lost in the slimes. The particle size measurement shows that over 90% of smithsonite particles in slime are less than 10μm. This range of particle size is difficult to recover by froth flotation.
5 CONCLUSIONS
1) A flowsheet that includes flotation of sulfide minerals, desliming, and sulphidization-flotation of oxide minerals for the treatment of lead-zinc oxide-sulfide mixed ores has been developed and the corresponding technology and reagent system to the flowsheet have been investigated in this research. The results of batch and continuous experiments indicate that the flowsheet is feasible and applicable, and all of the valuable minerals in the feed can be recovered in the processes. The results also suggest that sulphidization-flotation process is an effective and applicable technology for the recovery of oxide minerals, such as smithsonite and cerussite. It is shown that desliming prior to flotation of smithsonite and cerussite is essential for successful recovery of the minerals, and a desliming operation of two-stage hydrocyclone is well feasible for the treatment of lead-zinc oxide-sulfide type ores. A successive treatment of the cerussite flotation concentrate by shaking table is proposed to further improve Pb grade of the concentrate.
2) Experiments show that the dosage of sodium sulfide, pulp temperature, and collector type are crucial affecting factors on the recovery of smithsonite and cerussite. The dosage of sodium sulfide depends on the grade of smithsonite and cerussite in feed. For cerussite there is an appropriate dosage level of sodium sulfide, and the recovery of cerussite drops with the increase in the dosage beyond that level. Flotation recovery of smithsonite increases with the increase of sodium sulfide at low dosage and becomes insensitive to it at high dosage. The appropriate flotation temperature for smithsonite and cerussite is found to be 25-40℃. Amines are found effective collectors for the sulphidation-flotation of smithsonite.
3) Based on the flowsheet, a cerussite concentrate of 16.90% Pb and a smithsonite concentrate of 36.28% Zn are obtained from the tested sample under above conditions in the continuous tests.
REFERENCES
[1]Weast R C. Handbook of Chemistry and Physics (54th edition) [M]. Cleveland, Ohio: CRC Press, 1973-1974.
[2]Rinelli G, Marabini A M. Flotation of zinc and lead oxide-sulphide ores with chelating agents [A]. 10th International Mineral Processing Congress [C]. London, 1973. 493-521.
[3]Marabini A M, Alesse V, Barbaro M. New synthetic collectors for selective flotation of zinc and lead oxidized minerals [A]. Amsterdam B V. XVI International Mineral Processing Congress [C]. Stockholm, Sweden: Elsevier Science Publishers, 1988: 1197-1208.
[4]Rey M, De Merre P, Mancuso R, et al, Recent research and developments in flotation of oxidized ores of copper, lead and zinc [J]. Colo Sch Mines Q, 1961, 56(3): 163-175.
[5]Maurice Rey M B E, Hon F I M M. Memoirs of milling and process metallurgy(Ⅰ)—flotation of oxidized ores [J]. Trans Instn Min Metall (Sect C: Mineral Process Extr Metall), 1979, 88: C245-250.
[6]ZHANG Xin-ping. Application of new reagents for flotation of lead and zinc oxide ores [J]. Mining and Metallurgy, 1996, 5(3): 40-45. (in Chinese)
[7]Herrera Urbina R, Sotillo F J, Fuerstenau D W. Effect of sodium sulfide additions on the pulp potential and amyl xanthate flotation of cerussite and galena [J]. Int J Miner Process, 1999, 55(3): 157-170.
[8]Herrera Urbina R, Sotillo F J, Fuerstenau D W. Amyl xanthate uptake by natural and sulfide-treated cerussite and galena [J]. Int J Miner Process, 1998, 55(2): 113-128.
[9]ZHU Cong-jie, Study on the effects of ultrafine perlimonite on smithsonite flotation [J]. Nonferrous Metals (Mineral Processing), 2003, 5: 18-22. (in Chinese)
[10]Fuerstenau M C B J, Sabacky B J. On the natural floatability of surfides [J]. Int J Miner Process, 1981, 8: 79-84.
[11]Berglund G. Pulp chemistry in sulphide mineral flotation [J]. Int J Miner Process, 1991, 33: 21-31.
[12]Djendova S, Mehandjiski V. Study of the effects of acoustic vibration conditioning of collector and frother on flotation of sulphide ores [J]. Int J Miner Preocess, 1992, 34: 205-217.
[13]Persson I, Persson P, Valli M, et al. Reactions on sulfide mineral surfaces in connection with xanthate flotation studied by diffuse reflectance FTIR spectroscopy, atomic absorption spectrophotometry and calorimetry [J]. International Journal of Mineral Processing, 1991, 33: 67-81.
[14]WANG Dian-zuo, LIU Qing-gao. Separation flotation of sulfide minerals with thiocarbonyl collectors [J]. Journal of Central South Institute of Mining and Metallurgy, 1984, 3: 19-27. (in Chinese)
[15]Zhang H W. New flotation flowsheet for treatment of Fankou complex lead-zinc ore [A]. Miner Process Extr Metall Inst Min Metall [C]. London, UK, 1984. 13.
[16]Steve H. Froth flotation—the importance of the froth [J]. Mining Magazine, 1996, 7: 16-17.
[17]Marabini A M, Alesse V, Garbassi F. Role of sodium sulphide, xanthate and amine in flotation of lead-zinc oxidized ores [A]. Jones M J, Oblatt R, Reagents in the Mineral Industry [M]. The Institute of Mining and Metallurgy, 1984. 125.
[18]Fuerstenau M C, Miller J D, Kuhn M C. Chemistry of Flotation [M]. SME, New York, 1985.
[19]Massacci P, Belardi G, Bonifazi C. Heat of reaction at solid-liquid interfaces in flotation of lead and zinc oxidized minerals [A]. Jones M J, Oblatt R, Reagents in the Mineral Industry [M]. The Institute of Mining and Metallurgy, 1984. 137.
[20]Lazarov D, Alexandrova L, Nishkov I. Temperature influence on the flotation behavior of magnetite [A]. Progress in Mineral Processing Technology [C]. Demirel & Ersayin, Balkema, Rotterdam, 1994. 167-171.
[21]HU His-Keng, HUANG Wei, MAO Ju-fan, Theory and Technology of Flotation [M]. Changsha: Central South University of Technology Press, 1991.(in Chinese)
[22]ZHANG Xin-ping, ZHOU Xiu-ying, WANG Shu-qiu, et al. Study on new flotation technology of Lanping lead-zinc oxide ore [J]. Mining and Metallurgy, 1995, 4(3): 38-43. (in Chinese)
[23]WANG Shu-qiu, LOU Lin, ZHANG Xin-ping, et al. Study of flotation flowsheet of lead-zinc oxide ores [J]. Nonferrous Metals (Mineral Processing), 1998, 3: 17-20. (in Chinese).
Received date: 2005-03-03; Accepted date:2005-05-30
Correspondence: LI Guang-hui, Associate professor, PhD; Tel: +86-731-8830542; E-mail: liguangh@mail.csu.edu.cn
(Edited by YANG Bing)