Cu单掺杂和Cu/Al复合掺杂纳米氢氧化镍的结构和电化学性能
来源期刊:中国有色金属学报(英文版)2013年第2期
论文作者:包 杰 朱燕娟 庄义环 许庆胜 赵汝冬 刘泳林 钟浩良
文章页码:445 - 450
关键词:纳米Ni(OH)2;超声波辅助沉淀法;掺杂;电化学性能
Key words:nano-Ni(OH)2; ultrasonic-assisted precipitation; doping; electrochemical performance
摘 要:采用超声波辅助沉淀法制备Cu单掺杂和Cu/Al复合掺杂的纳米Ni(OH)2样品,测试样品的晶相结构、粒径、形貌、振实密度及电化学性能。结果表明,样品均具有α相结构且其平均粒度的分布范围窄,Cu单掺杂的纳米Ni(OH)2呈现不规则形态,而Cu/Al复合掺杂的纳米Ni(OH)2呈准球状且具有更大的振实密度。将纳米样品以8%的比例掺入到商业用微米级球形镍中制成混合电极。充放电和循环伏安测试结果表明,Cu/Al复合掺杂纳米Ni(OH)2的电化学性能优于Cu单掺杂的纳米Ni(OH)2的,前者的放电比容量最高达到330 mA·h/g (0.2C),比Cu单掺杂样品的高12 mA·h/g,比纯球镍电极的高91 mA·h/g。此外,Cu/Al复合掺杂纳米样品的质子扩散系数比Cu单掺杂样品的高52.3%。
Abstract: Nanometer Cu singly doped and Cu/Al co-doped nickel hydroxides were synthesized by ultrasonic-assisted precipitation method. Their crystal structure, particle size, morphology, tap density and electrochemical performance were investigated. The results show that the samples have α-phase structure with narrow particle size distribution. Cu singly doped nano-Ni(OH)2 contains irregular particles, while Cu/Al co-doped nano-Ni(OH)2 displays a quasi-spherical shape and has a relatively higher tap density. Composite electrodes were prepared by mixing 8% (mass fraction) nanometer samples with commercial micro-size spherical nickel. The charge/discharge test and cyclic voltammetry results indicate that the electrochemical performance of Cu/Al co-doped nano-Ni(OH)2 is better than that of Cu singly doped nano-Ni(OH)2, the former’s discharge capacity reaches 330 mA·h/g at 0.2C, 12 mA·h/g and 91 mA·h/g larger than that of Cu singly doped sample and pure spherical nickel electrode, respectively. Moreover, the proton diffusion coefficient of Cu/Al co-doped sample is 52.3% larger than that of Cu singly doped sample.
Trans. Nonferrous Met. Soc. China 23(2013) 445-450
Jie BAO, Yan-juan ZHU, Yi-huan ZHUANG, Qing-sheng XU, Ru-dong ZHAO, Yong-lin LIU, Hao-liang ZHONG
School of Physics and Optoelectronic Engineering, Guangdong University of Technology, Guangzhou 510006, China
Received 12 January 2012; accepted 2 August 2012
Abstract: Nanometer Cu singly doped and Cu/Al co-doped nickel hydroxides were synthesized by ultrasonic-assisted precipitation method. Their crystal structure, particle size, morphology, tap density and electrochemical performance were investigated. The results show that the samples have α-phase structure with narrow particle size distribution. Cu singly doped nano-Ni(OH)2 contains irregular particles, while Cu/Al co-doped nano-Ni(OH)2 displays a quasi-spherical shape and has a relatively higher tap density. Composite electrodes were prepared by mixing 8% (mass fraction) nanometer samples with commercial micro-size spherical nickel. The charge/discharge test and cyclic voltammetry results indicate that the electrochemical performance of Cu/Al co-doped nano-Ni(OH)2 is better than that of Cu singly doped nano-Ni(OH)2, the former’s discharge capacity reaches 330 mA·h/g at 0.2C, 12 mA·h/g and 91 mA·h/g larger than that of Cu singly doped sample and pure spherical nickel electrode, respectively. Moreover, the proton diffusion coefficient of Cu/Al co-doped sample is 52.3% larger than that of Cu singly doped sample.
Key words: nano-Ni(OH)2; ultrasonic-assisted precipitation; doping; electrochemical performance
1 Introduction
Nickel hydroxide has drawn considerable attention due to its application as the active material in the positive electrode for nickel-based rechargeable alkaline batteries. It has two polymorphs: α-Ni(OH)2 and β-Ni(OH)2 [1]. The β-Ni(OH)2 (interlayer separation of about 4.6 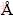 ) may transform into γ-NiOOH (interlayer separation of 7-8
) may transform into γ-NiOOH (interlayer separation of 7-8 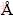 ) when overcharged, which results in large volume expansion and poor electric contact between the active material in electrode. Contrast to the β-Ni(OH)2/ γ-NiOOH cycle, the volumetric change between α-Ni(OH)2 and γ-NiOOH cycle is greatly reduced because of their similar lattice parameters [2]. However, the α-Ni(OH)2 is a metastable phase and easily changes to β-Ni(OH)2 in strong alkaline solution [3]. To synthesize stabilized α-Ni(OH)2, partial substitution of Ni ions in the nickel hydroxide lattice with Al [4], Y [5], Zn [6] or Co [7] ions has been extensively employed.
) when overcharged, which results in large volume expansion and poor electric contact between the active material in electrode. Contrast to the β-Ni(OH)2/ γ-NiOOH cycle, the volumetric change between α-Ni(OH)2 and γ-NiOOH cycle is greatly reduced because of their similar lattice parameters [2]. However, the α-Ni(OH)2 is a metastable phase and easily changes to β-Ni(OH)2 in strong alkaline solution [3]. To synthesize stabilized α-Ni(OH)2, partial substitution of Ni ions in the nickel hydroxide lattice with Al [4], Y [5], Zn [6] or Co [7] ions has been extensively employed.
Among these additives, Al is most used due to its good stability and low cost. It was reported that Cu shows a good effect on improving the utilization of active material and increasing the specific discharge capacity [8,9]. Besides, studies [10,11] have shown that composite doping may generate synergy effect, making the structure of α-Ni(OH)2 more stable than singly doping. So, the electrochemical performance of the electrodes can be improved effectively. In the present study, ultrasonic-assisted precipitation method was employed to synthesize Cu singly doped and Cu/Al co-doped α-Ni(OH)2. The morphology, particle size, tap density, crystal structure and electrochemical performance were measured, and the test results were discussed.
2 Experimental
2.1 Preparation and characterization of nickel hydroxide
The nano-Ni(OH)2 samples were prepared by an ultrasonic-assisted precipitation method at 50 °C. A mixed aqueous solution containing NiCl2·6H2O, CuCl2·2H2O, Al2(SO4)3·18H2O (the molar ratio of Ni to Cu to Al is 1:0.10:0.08) with Twain 80 as surfactant, and a proper amount of NaOH aqueous solution with anhydrous sodium carbonate and ammonia as buffer solution were dropped into a mother liquid under stirring.
The pH value was kept at 9.00±0.10 by pH meter. The agitation lasted 5 h after dropping was finished and the ultrasonic dispersing condition was employed in all above processes. Then the suspension was aged for 12 h at 50 °C, washed three times with deionized water and twice with anhydrous ethanol to eliminate impurities and then dried to constant mass at 80 °C (sample B). For comparison, the Cu singly doped nano-Ni(OH)2 (sample A, the molar ratio of Ni to Cu is 1:0.10) was prepared by the same method.
The crystal structure of the samples was determined on a D/Max-ⅢA X-ray diffractometer, with a Cu Kα radiation source (λ=1.54 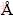 , 36.0 kV, 20 mA). TEM was operated on a Philips Tecnai 10 Transmission Electron Microscope to study the morphology of the samples. Particle size distribution (PSD) was measured using a Nanotrac 150 particle size analyzer.
, 36.0 kV, 20 mA). TEM was operated on a Philips Tecnai 10 Transmission Electron Microscope to study the morphology of the samples. Particle size distribution (PSD) was measured using a Nanotrac 150 particle size analyzer.
2.2 Preparation of nickel electrode and electro- chemical measurement
The positive electrode was a mixture of sample A or B (8%), commercial micro-size β spherical nickel (86%, obtained from Changsha Mining and Metallurgy Institute, China), nickel powder (3%) which was filled into a foam nickel sheet (2.5 cm×2.5 cm) using CMC (2%), and PTFE (1%) solution as binder. The electrode was dried at 80 °C for 20 min, and then mechanically pressed to a thickness of about 0.5 mm. The prepared positive electrodes were named as electrode Ae and Be. The negative electrode was hydrogen storage alloy. A mixture of 6 mol/L KOH and 15 g/L LiOH was used as the electrolyte. For comparison, the pure spherical nickel electrode was prepared by the same method and denoted as electrode Ce.
Cyclic voltammetry (CV) measurement of the electrodes was performed on an electrochemistry workstation (CHI 760D) with a counter electrode (nickel foam) and an Hg/HgO reference electrode. The test potential ranged from -0.2 to 0.7 V and the test scanning rate ranged from 0.02 to 0.10 V/s.
Charge/discharge measurement was done with the simulated batteries by using a Neware BTS-51800 battery testing instrument at room temperature. The simulated batteries were assembled with the prepared positive electrode, negative electrode and polypropylene as the separator. The electrodes were charged at 0.2C for 6 h, rested for 10 min, and then discharged at 0.2C to 1.0 V. In order to decrease the error, five electrodes were examined in the same situation.
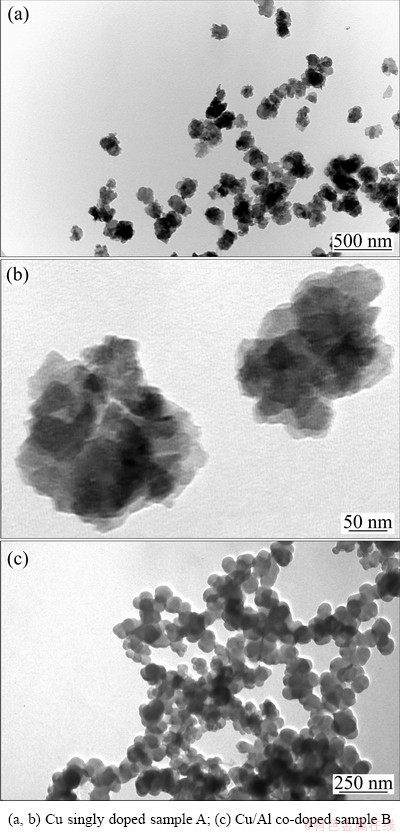
Fig. 1 TEM images of samples
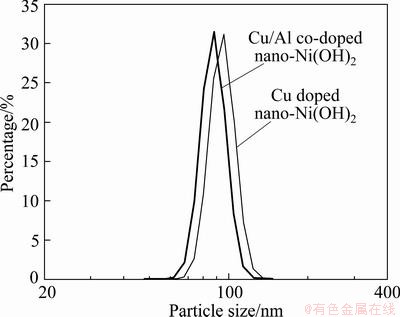
Fig. 2 Particle size distribution of samples
3 Results and discussion
3.1 Morphology, particle size and tap density of prepared samples
Figure 1 shows the TEM morphology of the prepared samples. It is seen that sample A contains irregular particles, the primary particles are 40-80 nm, and the secondary particles range from 80 to 140 nm. Sample B shows quasi-spherical structure with a smooth surface, and the diameter of primary particles is 60-80 nm. The particle size distribution (PSD) of the samples is shown in Fig. 2 and the corresponding average particle sizes are presented in Table 1. Although the primary particles of sample A are smaller than those of sample B, the larger secondary particles of sample A make its average particle size is higher than that of sample B.
Table 1 Tap density and average particle size of samples

Figures 1 and 2 also present that both samples have uniform particles with narrow particle size distribution. The uniform particles may be related to the application of ultrasonic and dispersant (Twain 80) in the preparation [12-14]. Due to the addition of Twain 80 in solution, every particle is almost in a same microenvironment under high frequency vibration of ultrasonic, and thus the particle sizes are uniform. Therefore, the synergy of ultrasonic and dispersant play a good role in obtaining well dispersed suspensions and controlling the particle size.
The tap density of nickel hydroxide is an important parameter indexing the material properties, which is associated with the performance of the positive electrodes [15]. The tap density of the samples which was tested by shock method is presented in Table 1. It can be seen that the average particle size of sample B is smaller than that of sample A, but contrary to the tap density. This is mainly caused by the fact that Cu/Al co-doped Ni(OH)2 is quasi-spherical, but irregular shape for Cu singly doped sample. When samples A and B are mixed with micro-size spherical nickel, sample B can more fully fill the gap in commercial micro-size spherical nickel, which is conductive to promoting the active material utilization. So, sample B is expected to get much better electrochemical performance.
3.2 Crystal structure
X-ray diffraction patterns of the samples are given in Fig. 3. Both samples display diffraction peaks at the angles 11°, 23°, 34°, 38°, 60°, which are in accordance with those of the α-Ni(OH)2 (JCPDS 38—0715). The asymmetrically broad diffraction peaks at 34°-38° are the characteristic of the turbostratic disorder in α-Ni(OH)2 [16].
It is also seen that all the peaks of sample B shift to the right as compared with those of sample A. This result can be attributed to the similar radius of Cu2+ and Ni2+, and the Cu2+ intercalation does not have significant effect on the crystal structure. Partial substitution of nickel ions with Al and Cu will result in a lattice deformation due to the smaller radius of Al3+, and the interlamellar spacing accordingly reduces. According to the Bragg equation, 2dsinθ=nλ, when the d value decreases, the value of θ will increase to keep nλ invariable, thus the diffraction peaks of sample B move to the right. The interplanar spacings (calculated from the Bragg equation) of two samples at (003), (006) and (110) planes are listed in Table 2. It is seen that sample B has a smaller interplanar spacing than sample A. In addition, the intensities of the peaks (003), (006) for sample B are higher than those of sample A, which indicates an increase in crystallinity upon Cu/Al co-doped sample.
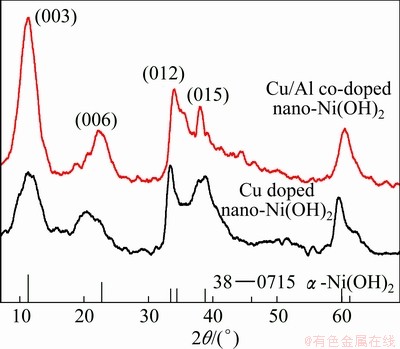
Fig. 3 XRD patterns of samples
Table 2 Interplanar spacing of two samples

3.3 Charging-discharging performance of electrodes
Figure 4 shows the typical charge-discharge curves of the electrodes Ae (Cu doped α-Ni(OH)2 electrode), Be (Cu/Al co-doped α-Ni(OH)2 electrode) and Ce (pure spherical nickel electrode) at a charge-discharge rate of 0.2C. The specific discharge capacity of electrode Be reaches 330 mA·h/g, which is 12 mA·h/g higher than that of electrode Ae (318 mA·h/g) and 91 mA·h/g higher than that of electrode Ce (239 mA·h/g). Compared with electrode Ae, electrode Be not only displays higher discharge capacity, but also shows a lower charge potential platform and a higher discharge potential platform. The higher specific capacity of electrode Be may be caused by the fact that introducing Al into the nickel hydroxide lattice may cause excess positive charge, so more anions and water molecules can be intercalated to the interlayer of nickel hydroxide in order to keep charge neutrality, resulting in an increase of defects. These defects is beneficial for protons intercalation and deintercalation in the slab of Cu/Al co-doped α-Ni(OH)2 [17], and more defects can also help the electrolyte to get into the interior of Ni(OH)2 to increase the conductivity. So electrode Be has a higher specific capacity than electrode Ae.
The cyclic performance of the electrodes Ae and Be at 0.2C rate is shown in Fig. 5. Cycle life is a significant index for all the second batteries. MH-Ni batteries may suffer from capacity loss during cycle as a result of deterioration of nickel hydroxide [18]. It is seen from the figure that the electrode Be shows a higher specific capacity and better cycling stability than the electrode Ae during the cycle. To quantitatively characterize the cyclic stability of the nickel electrodes, the deterioration rate Rd is used. The Rd values of electrodes Ae and Be are 9.10% and 6.07%, respectively, after 40 cycles. This indicates that Cu/Al co-doped nano-Ni(OH)2 has much better cyclic stability and higher discharge capacity than Cu singly doped nano-Ni(OH)2.
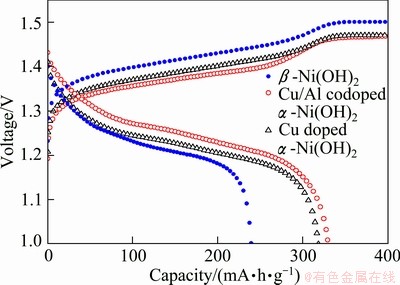
Fig. 4 Charge-discharge curves of electrodes at 0.2C rate
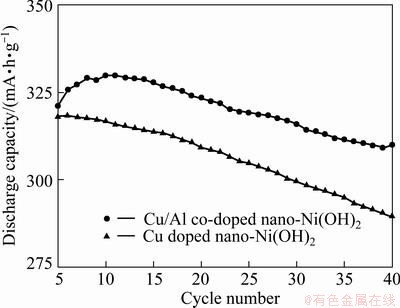
Fig. 5 Cyclic performance of electrodes at 0.2C discharge rate
3.4 Cyclic voltammetric behavior
The typical CV curves of electrodes Ae and Be at various scanning rates are presented in Fig. 6. For both electrodes, as the scanning rate increases, the anodic and cathodic peak potentials shift to more positive and more negative direction, respectively. Figure 7 gives the CV curves of the electrodes Ae and Be at the scanning rate of 0.06 V/s and the cyclic voltammetric parameters are listed tabulated in Table 3. The potential difference (ΔφO,R) between the anodic (φO) and cathodic (φR) position was used to index the reversibility of the electrode reaction. The smaller the ΔφO,R is, the more reversible the redox reaction is [19]. It is noticed that electrode Be has a smaller ΔφO,R than electrode Ae, indicating that the addition of Al can improve the reversibility of the electrode reaction. Meanwhile, more active ingredients can be utilized during charge- discharge process.
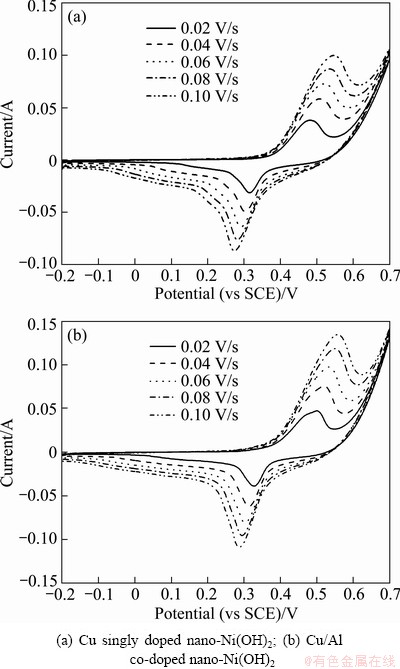
Fig. 6 Cyclic voltammograms of two electrodes at various scanning rates
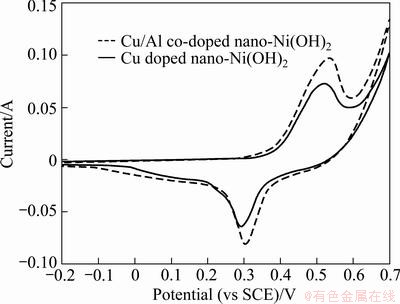
Fig. 7 Cyclic voltammograms of two electrodes at scanning rate of 0.06 V/s
Table 3 Results of cyclic voltammetry measurements

It is also seen from Table 3 that the difference (φOER-φO) between φOER and φO was almost the same, which is in accordance with the charge–discharge results shown in Fig. 4 (charge curve has two platforms, oxidation reaction the electrode occurs in the easier stage of charging, but oxygen evolution reaction in the later stage of charging). The difference (φOER-φO) shows the difficulty degree of electrodes for oxygen evolution. The large value indicates that higher charging efficiency is expected [20]. The result demonstrates that there is no obvious difference between the electrodes Ae and Be, indicating that the addition of Al does not have significant effect on improving the charge efficiency and restraining the oxygen evolution reaction.
The relationship between oxidation peak current Ip and square root of scanning rate υ1/2 is present in Fig. 8. Generally, in semi-infinite diffusion conditions, the relationship between Ip and v1/2 is linear at 25 °C. From Fig. 8, the electrode reaction of both electrodes is controlled by semi-infinite diffusion conditions, which agrees with the reaction mechanism of nickel hydroxide electrode.
According to Randle-Sevick equation [21]:
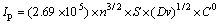 (1)
(1)
where n is the electron number of reaction (the value is about 1 for nickel hydroxide); S is the real surface area of the electrode; D is the diffusion coefficient; and C0 is the initial concentration of the active material. For nickel hydroxide electrode, the C0 value is equal to the proton concentration (H+) in solid state because the electrode reaction is Ni(OH)2 NiOOH+H+, and C0 can be expressed as C0=ρ/M, where ρ is the theoretical density of nickel hydroxide, 2.82 g/cm3; M is the mole mass of nickel hydroxide, 92.7 g/mol.
NiOOH+H+, and C0 can be expressed as C0=ρ/M, where ρ is the theoretical density of nickel hydroxide, 2.82 g/cm3; M is the mole mass of nickel hydroxide, 92.7 g/mol.

Fig. 8 Relationship between anodic peak current and square root of scanning rate for electrodes
The diffusion coefficients D of both electrodes can be calculated from the slope of the fitted line in Fig. 8 and Eq. (1). The proton diffusion coefficient of electrode Be is 1.31×10-10 cm2/s, which is larger than that of electrode Ae (0.86×10-10 cm2/s), indicating that the substitution of Al can accelerate the proton diffusion rate in nickel hydroxide. It can be attributed to the fact that sample B is quasi-spherical with good liquidity and more defects in it can provide more channels for protons diffusion in redox process.
4 Conclusions
1) Cu singly doped (sample A) and Cu/Al co-doped nanometer α-Ni(OH)2 (sample B) were prepared by ultrasonic-assisted precipitation method. The primary particle size of sample A is 40-80 nm with irregular shape, the secondary particle size is 80-140 nm. The primary particle size of sample B is 60-80 nm with quasi-spherical structure. And sample B shows a little higher tap density than sample A.
2) Cu/Al co-doped α-Ni(OH)2 has a higher discharge capacity, better cyclic stability, higher electrochemical reaction reversibility and larger proton diffusion coefficient than Cu singly doped α-Ni(OH)2. The electrode with 8% sample B displays a capacity of 330 mA·h/g at 0.2C, which is 12 and 91 mA·h/g larger than that of electrode with 8% sample A and pure spherical nickel electrode, respectively.
3) The addition of Al can improve the electrode reaction reversibility and accelerate the proton diffusion in nickel hydroxide (the proton diffusion coefficient of Cu/Al co-doped α-Ni(OH)2 is 52.3% larger than that of Cu singly doped sample), but has no significant effect on improving the charging efficiency and inhibiting oxygen evolution reaction.
References
[1] WANG Y X, HU Z A, WU H Y. Preparation and electrochemical performance of alpha-nickel hydroxide nanowire [J]. Materials Chemistry and Physics, 2011, 126(3): 580–583.
[2] LIU C J, LI Y W, LI P P, XING C X. Structure and electrochemical performance of nickel hydroxide synthesized by rapid quench method [J]. Materials Research Bulletin, 2010, 45(12): 2001-2005.
[3] ZHANG Q, XU Y H, WANG X L. The electrochemical behavior of Ni(OH)2 in KOH solution containing aluminum hydroxide [J]. Materials Chemistry and Physics, 2004, 86(2-3): 293-297.
[4] WANG Hong, TANG Zhi-yuan, LIU Yuan-gang, LEE Chang-sheng. Synthesis and behavior of Al-stabilized α-Ni(OH)2 [J]. Transactions of Nonferrous Metals SocietyofChina, 2009, 19(1): 170-175.
[5] YE Xian-cong, ZHU Yan-juan, ZHANG Zhong-ju, WU Shang-gai, ZHOU Zhuo-jun, ZHENG Han-zong, LIN Xiao-ran. Study on the preparation and electrochemical performance of rare earth doped nano-Ni(OH)2 [J]. Journal of Rare Earths, 2011, 29(8): 787-792.
[6] GAO X R, LEI L X, HU M, QIN L M, SUN Y M. Morphology and electrochemical performance of Zn-doped [Ni4Al(OH)10]OH [J]. Journal of Power Sources, 2009, 191(2): 662-668.
[7] NETHRAVATHI C, RAVISHANKAR N, SHIVAKUMARA C, RAJAMATHI M. Nanocomposites of alpha-hydroxides of nickel and cobalt by delamination and co-stacking: Enhanced stability of alpha-motifs in alkaline medium and electrochemical behavior [J]. Journal of Power Sources, 2007, 172(2): 970-974.
[8] YU Dan-mei, CHEN Chang-guo, ZHOU Shang-qi, YANG Yu-qiong. Modification study of nano-Ni(OH)2 doped copper [J]. Battery Bimonthly, 2005, 35(2): 102-104.
[9] LIU Chang-jiu, WANG Hui-jing, LU Li-jun. Preparation and electrochemical performances investigation of Cu-substituted amorphous nano Ni(OH)2 [J]. Nano-processing Technique, 2006, 3(5): 42-46.
[10] CHEN Hui, WANG Jian-ming, PAN Tao, XIAO Hui-ming, ZHANG Jian-qing. Structure and electrochemical performance of Al and Zn co-substituted α-Ni(OH)2 [J]. Transactions of Nonferrous Metals Society ofChina, 2003, 13(1): 85-90.
[11] ZHANG Z J, ZHU Y J, BAO J, ZHOU Z J, ZHENG H Z, LIN X R. Electrochemical performance of multi-element-doped α-nickel hydroxide prepared by supersonic co-precipitation method [J]. Journal of Alloys and Compounds, 2011, 509(25): 7034-7037.
[12] ZHOU Huan-bo, ZHOU Zhen-tao. Effects of ultrasonic treatment on the structure and electrochemical performances of spherical beta-Ni(OH)2 [J]. Chinese Journal of Chemistry, 2006, 24(1): 37-44.
[13] ZHANG Zhong ju, ZHU Yan juan, BAO Jie, ZHOU Zhuo jun, YE Xian cong, XUQing sheng. Effect of ultrasonic on the structure and electrochemical performance of α-Ni(OH)2 electrodes [J]. TransactionsofNonferrousMetals SocietyofChina, 2011, 21(12): 2654-2659.
[14] CABANAS-POLO S, SUSLICK K S, SANCHEZ-HERENCIA A J. Effect of reaction conditions on size and morphology of ultrasonically prepared Ni(OH)2 powders [J]. Ultrasonics Sonochemistry, 2011, 18(4): 901-906.
[15] ZHANG W G, JIANG W Q, YU L M, FU Z Z, XIA W, YANG M L. Effect of nickel hydroxide composition on the electrochemical performance of spherical Ni(OH)2 positive materials for Ni–MH batteries [J]. International Journal of Hydrogen Energy, 2009, 34(1): 473-480.
[16] YANG D N, WANG R M, HE M S, ZHANG J, LIU Z F. Ribbon- and board like nanostructures of nickel hydroxide: Synthesis, characterization, and electrochemical properties [J]. Journal of Physical Chemistry, 2005, 109(16): 7654-7658.
[17] RAMESH T N, KAMATH P V. Planar defects in layered hydroxides: Simulation and structure refinement of beta-nickel hydroxide [J]. Materials Research Bulletin, 2008, 43(12): 3227-3233.
[18] HAN X J, XU P, XU C Q, ZHAO L, MO Z B, LIU T. Study of the effects of nanometer β-Ni(OH)2 in nickel hydroxide electrodes [J]. Electrochimica Acta, 2005, 50(14): 2763-2769.
[19] XIE Shou-yun, FANG Qing, CHENG Yan, JIAN Xu-yu, ZHU Lei, WANG Zhong, YU Hai-jun. Effects of erbium added by mechanically mixture and surface coating on high temperature performances of nickel hydroxide electrode [J]. Journal of the Chinese Rare Earth Society, 2008, 26(5): 570-576. (in Chinese)
[20] LI Y W, YAO J H, LIU C J, ZHAO W M, DENG W X, ZHONG S K. Effect of interlayer anions on the electrochemical performance of Al-substituted α-type nickel hydroxide electrodes [J]. International Journal of Hydrogen Energy, 2010, 35(6): 2539-2545.
[21] CORRIGAN D A, BENDERT R M. Effect of coprecipitated metal ions on the electrochemistry of nickel hydroxide thin films: Cyclic voltammetry in 1 M KOH [J]. Journal of the Electrochemical Society, 1989, 136(3): 723-728.
包 杰,朱燕娟,庄义环,许庆胜,赵汝冬,刘泳林,钟浩良
广东工业大学 物理与光电工程学院,广州 510006
摘 要:采用超声波辅助沉淀法制备Cu单掺杂和Cu/Al复合掺杂的纳米Ni(OH)2样品,测试样品的晶相结构、粒径、形貌、振实密度及电化学性能。结果表明,样品均具有α相结构且其平均粒度的分布范围窄,Cu单掺杂的纳米Ni(OH)2呈现不规则形态,而Cu/Al复合掺杂的纳米Ni(OH)2呈准球状且具有更大的振实密度。将纳米样品以8%的比例掺入到商业用微米级球形镍中制成混合电极。充放电和循环伏安测试结果表明,Cu/Al复合掺杂纳米Ni(OH)2的电化学性能优于Cu单掺杂的纳米Ni(OH)2的,前者的放电比容量最高达到330 mA·h/g (0.2C),比Cu单掺杂样品的高12 mA·h/g,比纯球镍电极的高91 mA·h/g。此外,Cu/Al复合掺杂纳米样品的质子扩散系数比Cu单掺杂样品的高52.3%。
关键词:纳米Ni(OH)2;超声波辅助沉淀法;掺杂;电化学性能
(Edited by Hua YANG)
Foundation item: Project (10774030) supported by the National Natural Science Foundation of China; Project (S2012010009955) supported by the Guangdong Province Natural Science Foundation of China; Project (12C232111916) supported by the Science and Technology Program of Guangzhou City of China
Corresponding author: Yan-juan ZHU; Tel: +86-13610281143; E-mail: zhuyjgz@126.com
DOI: 10.1016/S1003-6326(13)62483-8

